Seelos to Test Potential Parkinson’s Treatment Targeting Alpha-synuclein Clumps
Seelos Therapeutics has acquired an exclusive license to further develop a treatment approach called SLS-007 for Parkinson’s disease which is intended to lessen the aggregation of alpha-synuclein protein.
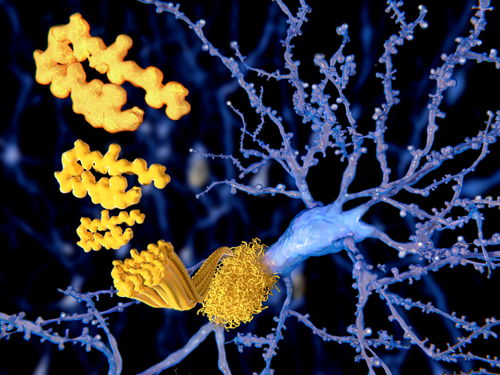
The therapeutic strategy was developed by scientists at University of California, Los Angeles, and includes a family of peptide blockers that target the build-up of alpha-synuclein — a major constituent of Lewy bodies found in Parkinson’s patients.
SLS-007 has shown an ability to slow disease progression in preclinical studies, including being able to stop alpha-synuclein propagation and seeding — a process in which a “seed” provides the template for the aggregation of normal protein into clumps — when using fibril preparations and alpha-synuclein seeds collected from patients with Parkinson’s or Lewy body dementia.
It specifically targets a central region of alpha-synuclein called the non-amyloid component core, which is particularly prone to aggregation.
Seelos will assess whether SLS-007 can be delivered in a mouse model of Parkinson’s, while also aiming to establish its in vivo (in the body) pharmacological profile and parameters of target engagement (to alpha-synuclein). Besides Parkinson’s, the company plans to test its approach on other neurological disorders.
“Accumulation and aggregation of (alpha-synuclein) is a pathological hallmark of (Parkinson’s),” Raj Mehra, PhD, Seelos’ chairman, founder, and CEO, said in a press release.
The Parkinson’s Disease News Today forums are a place to connect with other patients, share tips and talk about the latest research. Check them out today!
Although its role is not completely understood, alpha-synuclein clumping appears pivotal in the development of Parkinson’s, Lewy body dementia, and multiple system atrophy; therefore, “reducing the levels of pathological forms of (alpha-synuclein) may alter the course of (Parkinson’s),” Mehra said.
Tim Whitaker, MD, Seelos’ head of research and development, noted the need to develop an improved way to restore the levels of dopamine in the striatum — a key brain area for motor control — as well as a safe and effective therapy to slow disease progression in Parkinson’s, given the lack of disease-modifying treatments and the side effects of long-term use of dopaminergic treatments.
“If we are successful in our planned preclinical and future clinical studies, SLS-007 may prove to be such a treatment,” Whitaker said.
The intellectual property to these peptides was owned by The Regents of the University of California. As part of the agreement, Seelos made an upfront payment of $100,000 to The UC Regents/UCLA. The company will also pay royalties if the therapy reaches market.