Advanced Brain Monitoring Earns ‘Most Innovative Medical Device Company’ Award
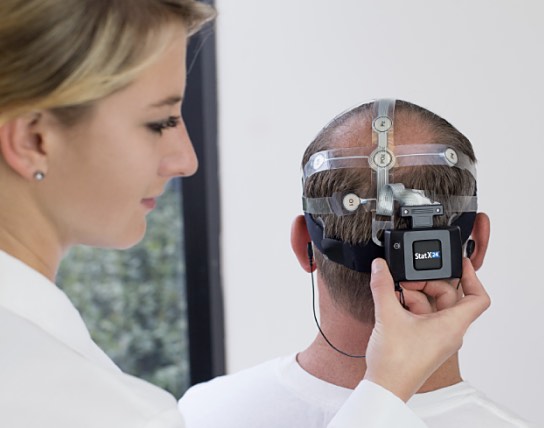
 Advanced Brain Monitoring, Inc. (ABM) has been declared the Most Innovative Medical Device Company by trade magazine Global Health and Pharma’s 2016 Healthcare and Pharmaceutical awards committee. The Carlsbad, Calif.-based medical technology company’s portfolio includes technology that can help the assessment of brain impairment in Parkinson’s disease.
Advanced Brain Monitoring, Inc. (ABM) has been declared the Most Innovative Medical Device Company by trade magazine Global Health and Pharma’s 2016 Healthcare and Pharmaceutical awards committee. The Carlsbad, Calif.-based medical technology company’s portfolio includes technology that can help the assessment of brain impairment in Parkinson’s disease.
“This award recognizes our company’s successes in developing technologies which enable clinicians and clinical trial sponsors to profile brain health through the analysis of the brain’s electrical activity (EEG) during sleep and wake,” ABM chief executive officer and co-founder Chris Berka said in a press release.
 The awards seek to recognize and chronicle the individuals, departments and organizations, corporate and public, working tirelessly throughout the industry. Commenting on the awards, GHP Coordinator Naomi Douglas stated in a press release that “The healthcare and pharmaceuticals market is the lifeblood of our society, and therefore it is a true honor to put the spotlight on our deserving winners … ”
The awards seek to recognize and chronicle the individuals, departments and organizations, corporate and public, working tirelessly throughout the industry. Commenting on the awards, GHP Coordinator Naomi Douglas stated in a press release that “The healthcare and pharmaceuticals market is the lifeblood of our society, and therefore it is a true honor to put the spotlight on our deserving winners … ”
 GHP stresses that its award winners are chosen solely on merit, in recognition and commendation of their ingenuity and hard work. For more information about GHP award winners, and insight into working practices of the health and pharma industries’ “best of the best,” you can access the winners’ supplemental information on the GHP website
GHP stresses that its award winners are chosen solely on merit, in recognition and commendation of their ingenuity and hard work. For more information about GHP award winners, and insight into working practices of the health and pharma industries’ “best of the best,” you can access the winners’ supplemental information on the GHP website
As a leader in neuroscience application development of devices for measuring and interpreting brain function, detecting abnormal neuro-cardio respiratory response during sleep, improving sleep quality, and enhancing performance for 15 years, Advanced Brain Monitoring has received research funding from the National Institute of Health (NIH). That included the recent award of a $1.5 million grant to expand its database of awake and sleeping EEG in patients with neurodegenerative diseases.
ABM research also has been funded variously by the Department of Defense Advanced Research Projects Agency (DARPA) and other government agencies. The company’s research findings have been reported in more than 100 scientific journals and papers, and its discoveries have been granted 27 patents.
 “The envisioned future is for routine brain health assessments during sleep and waking to be conducted in a manner similar to a mammogram or colonoscopy,” CEO Berka observed. “Ultimately, early detection will increase the likelihood that an intervention can be matched to the patient based on the presence of brain biomarkers and administered prior to the onset of cognitive decline.”
“The envisioned future is for routine brain health assessments during sleep and waking to be conducted in a manner similar to a mammogram or colonoscopy,” CEO Berka observed. “Ultimately, early detection will increase the likelihood that an intervention can be matched to the patient based on the presence of brain biomarkers and administered prior to the onset of cognitive decline.”
Six FDA-cleared, CE-marked ABM medical systems are currently distributed in North America, Europe, Asia, and Australia. ABM-designed medical devices have been used in assessing and treating more than 1.5 million patients worldwide.
Services offered by ABM include streamlined EEG acquisition, secure transmission via a cloud portal with rapid analyses and reporting. They also can provide proprietary functional Neuro-Electrophysiological Imaging (fNEI) that simultaneously measures EEG during resting state, with or without concurrent neurocognitive tests that activate neural circuits involved in brain functions such as attention, memory and emotion. Quantified data from these tests can be compared to a database containing results from more than 10,000 test sessions on both healthy and impaired individuals.
 “Preliminary results indicate potential for estimating the severity of brain impairment for Alzheimer’s and Parkinson’s disease, mild cognitive impairment, and Frontotemporal dementia,” said ABMs’ Chief Medical Officer, Philip Westbrook, a clinical professor of medicine at UCLA. Westbrook has been actively involved in professional organizations associated with sleep disorders, and has served as president of the American Sleep Disorder Association (ASDA).
“Preliminary results indicate potential for estimating the severity of brain impairment for Alzheimer’s and Parkinson’s disease, mild cognitive impairment, and Frontotemporal dementia,” said ABMs’ Chief Medical Officer, Philip Westbrook, a clinical professor of medicine at UCLA. Westbrook has been actively involved in professional organizations associated with sleep disorders, and has served as president of the American Sleep Disorder Association (ASDA).
ABM’s Sleep Profiler device monitors brain function during sleep and is able to detect biomarkers associated with neuro-degeneration and chronic diseases. Clinical applications of Sleep Profiler include objective assessment of prescription sleep aid effectiveness in patients with insomnia and the impact of anti-depressants and  anti-hypertensive medications have on quality sleep. “Sleep Profiler biomarkers have also been associated with the brain patterns of intensive care unit (ICU) patients who had sepsis or died,” said ABM President Daniel J. Levendowski. “Sleep Profiler will soon enable real-time monitoring of abnormal sleep patterns in ICU patients.”
anti-hypertensive medications have on quality sleep. “Sleep Profiler biomarkers have also been associated with the brain patterns of intensive care unit (ICU) patients who had sepsis or died,” said ABM President Daniel J. Levendowski. “Sleep Profiler will soon enable real-time monitoring of abnormal sleep patterns in ICU patients.”
Advanced Brain Monitoring also offers clinical trial services, specializing in early phase investigations of neurodegenerative and psychiatric indications, and the company can provide a comprehensive suite of EEG based sleep and daytime assessment technologies for use in pharmaceutical, medical device and academic clinical trials. ABM’s expertise spanning a broad range of neurological disorders enables its neuroscience research team to deliver streamlined EEG acquisition, secure data integrity management, customized data analysis, and worldwide clinical support