FAQs about Ropinirole
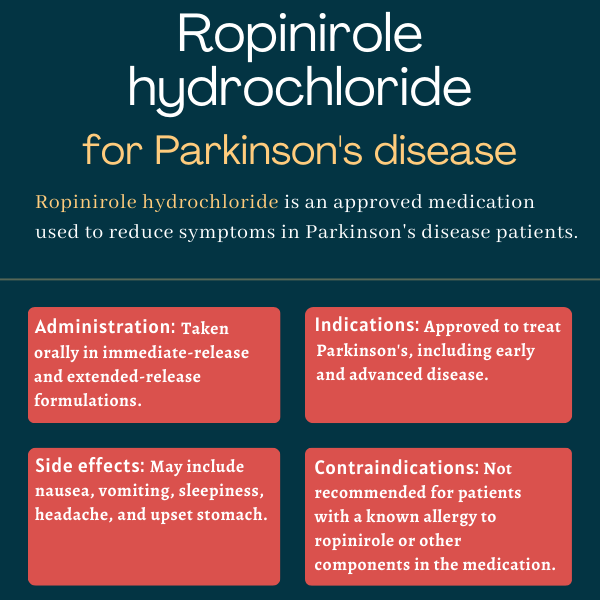
The U.S. Food and Drug Administration first approved ropinirole immediate-release tablets more than a quarter century ago, in September 1997, to treat people with Parkinson’s disease. The extended-release formulation was approved in July 2008 for the same indication.
Animal studies suggest ropinirole may be harmful to a developing fetus. However, its effects on pregnant patients are unknown due to a lack of clinical data, so it is unclear whether the therapy can be safely used in this population. It is recommended that patients consult their doctor if they plan to become pregnant while taking ropinirole.
Some people taking ropinirole may experience extreme drowsiness or unexpected episodes of falling asleep during daily activities. It is advised that patients starting this treatment avoid driving or engaging in potentially dangerous activities until they understand how the medication affects them. If these adverse events occur, it is recommended that patients talk to their doctor.
Based on data from clinical trials, ropinirole induces significant reductions in off time in people with advanced Parkinson’s disease as early as two weeks after starting treatment. However, because the initial recommended dose of ropinirole may not work for everyone, some patients may require dose adjustments before experiencing the full benefits of the therapy.
While neither hair loss nor weight have been reported as side effects of ropinirole in clinical trials, dopamine agonists may increase the risk of developing compulsive behaviors such as binge eating, which may lead to weight gain. Reversible hair loss also was occasionally reported in patients receiving ropinirole treatment. Patients should talk with their healthcare team if they experience any unanticipated effects of treatment.
Related Articles
 Fact-checked by
Fact-checked by