Deterioration of Nerve Cell Structure Not the Main Cause of Early Parkinson’s Symptoms, Mouse Study Suggests
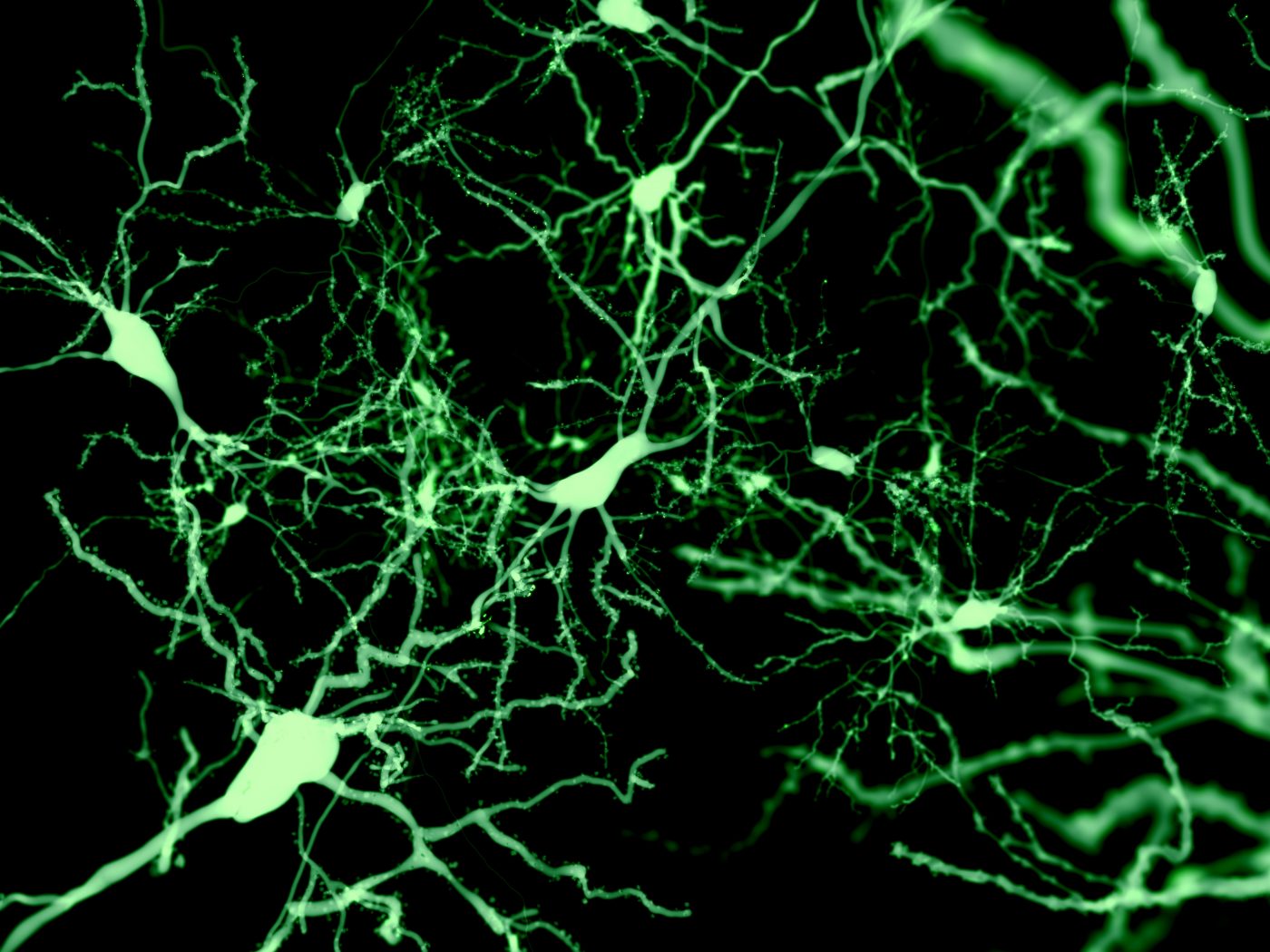
Although the structure of dopaminergic neurons gradually deteriorates before cell death, these alterations do not seem to account for the subtle impairments seen during the early stages of Parkinson’s disease, a mouse study has found.
The study, “Progressively Disrupted Somatodendritic Morphology in Dopamine Neurons in a Mouse Parkinson’s Model,” was published in Movement Disorders.
Parkinson’s is a progressive neurodegenerative disorder caused by the gradual loss of dopaminergic neurons in the substantia nigra, a region of the brain responsible for movement control.
Previous studies in animal models have shown that neuron dysfunction and cell death start to occur before animals display noticeable motor symptoms associated with Parkinson’s. However, the precise morphological (structural) and functional changes that occur in neurons at this early stage of disease are still not very well understood.
Researchers from the University of Texas Health used a mouse model of adult-onset parkinsonism that perfectly mirrors “the slow, progressive course of Parkinson’s seen in the majority of patients,” according to the study.
These mice, called MitoPark, lack the mitochondrial transcription factor A (TFAM) gene specifically in dopaminergic neurons. As a result, at 12 weeks of age, they show a marked decrease of brain innervation in the striatum — a region responsible for motor coordination — followed by neuron cell death at 30 weeks old.
To analyze the morphology of individual neurons in brain slices from MitoPark mice during the early stages of disease, researchers used a technique called whole-cell patch clamp — a technique that allows the study of the electrical properties of neurons — together with fluorescent labeling.
At 16 weeks of age, these animals’ dopaminergic neurons were significantly reduced in size with a lower number of branching points in dendrites — the long, slender projections of a neuron that carry an electrical signal from the cell body to the point of contact with another neuron, called the synapse.
“Alterations in somatic [cell body] and dendritic structure are likely to have direct effects on dopamine-dependent motor function and reward learning. In these neurons, dendrites serve important roles as sites for synaptic termination and contribute to many determinants of cell excitability,” the researchers said.
These defects worsened significantly as animals got older, from 16 to 31 weeks of age, eventually leading to neuronal death.
“Dendritic branching and soma size, although intact in dopamine neurons from 12-week-old MitoPark mice, are progressively and severely disrupted in a manner that undoubtedly impairs cellular function in advance of neuronal death,” the researchers wrote.
Although this decline in neuron morphology occurred at a similar rate in animals from both sexes, it did not begin until after the age at which the first mild locomotor and learning alterations start to occur (approximately 12 weeks).
As a result, the progressive and severe decline in neuronal morphology that occurs prior to cell death does not seem to be involved in the initial motor and cognitive impairments observed in this mouse model.
“This work could help identify the ideal time window for specific treatments to halt disease progression and avert debilitating motor deficits in Parkinson’s patients,” the researchers concluded.






