TOM20 Protein Rescues Nerve Cells Damaged in Parkinson’s in Rat Study
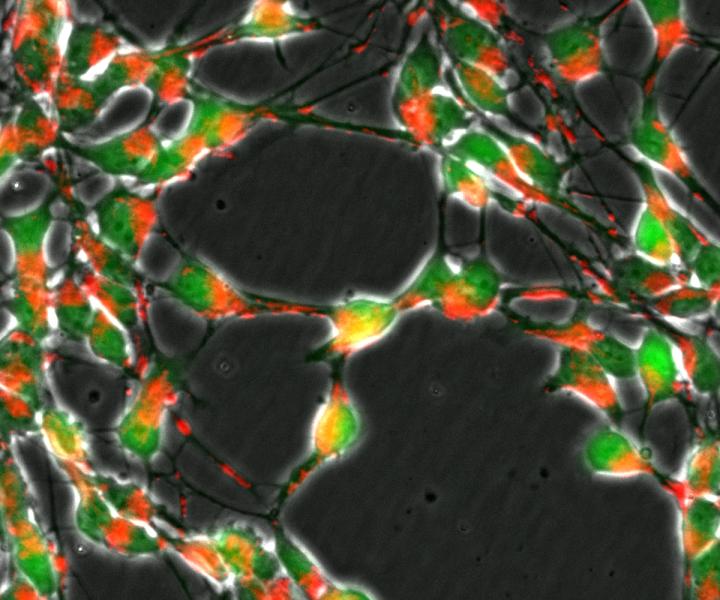
Loss of the DJ1 gene in nerve cells (green) impairs the transport of mitochondria (red) and important metabolic pathways of the cellular respiration. CREDIT University of Luxembourg
Production of the protein TOM20 rescued nerve cells that are damaged in people with Parkinson’s disease due to the toxic buildup of alpha-synuclein protein, a study in rats revealed.
According to researchers, the interaction between TOM20 and alpha-synuclein represents a potential therapeutic target for disease intervention.
The study, “Protection from α-Synuclein induced dopaminergic neurodegeneration by overexpression of the mitochondrial import receptor TOM20,” was published in the journal Nature NPJ Parkinson’s Disease.
A hallmark of Parkinson’s disease is the toxic buildup of the protein alpha-synuclein within nerve cells (neurons) that produce dopamine — a chemical messenger or neurotransmitter involved in motivation, reward, attention, memory, and regulating body movements.
At the same time, these neurons have malfunctioning mitochondria — small structures within cells that generate energy.
To properly function, mitochondria need to import almost all of the proteins they contain. To do so, they depend on a receptor called TOM20. Recent evidence suggests that alpha-synuclein buildup, as occurs in Parkinson’s, directly interacts with TOM20, blocking the import of other proteins and contributing to the death of dopamine-producing neurons (dopaminergic neurons).
Stopping this blockade may be a therapeutic strategy to rescue dopaminergic neurons early in disease development.
To this end, researchers at the University of Pittsburgh conducted a study examining the interaction of alpha-synuclein and mitochondrial function within dopaminergic neurons.
The team injected adult male rats (under anesthesia) with a harmless adeno-associated virus-2 (AAV2) that overproduced human TOM20 directly into the area of the brain with dopaminergic neurons. That brain area, called the substantia nigra, regulates balance and movement and is most affected in Parkinson’s.
The protein was produced in parallel with human alpha-synuclein (AAV2-TOM20/alphaSyn) or a control protein called GFP (AAV2-TOM20/GFP). As a disease control, alpha-synuclein was generated with the GFP protein (AAV2-alphaSyn/GFP). After infusion, the rats were monitored over 12 weeks.
Rats infused with either of these AAV2 vectors producing alpha-synuclein (AAV2-TOM20/alphaSyn or AAV2-alphaSyn/GFP) showed robust expression of the protein within the neurons of the substantia nigra, which was not seen in AAV2-TOM20/GFP treated control rats. Midbrain tissue analysis found some of the alpha-synuclein protein had formed toxic clumps (aggregates).
Animals given either AVV2 vector with the protein had a fivefold increase in TOM20 levels within dopaminergic neuron cell bodies over rats that received the disease control vector AAV2-alphaSyn/GFP.
No significant changes in motor abilities were observed between all infused animals during the 12-week study.
Brain section analysis found that rats that received the disease control vector (AAV2-alphaSyn/GFP) had about a 40% reduction in nerve fibers. In contrast, those given the protein vector with alpha-synuclein (AAV2-TOM20/alphaSyn) showed no loss of nerve fibers.
Likewise, dopaminergic neurons within the substantia nigra were significantly depleted by 47% in rats infused with the disease control vector. However, co-production with TOM20 protected against alpha-synuclein-induced dopaminergic neuron cell death.
Infusion with the disease vector also reduced up to 90% several proteins within the mitochondria. Conversely, dopaminergic neurons that overexpressed both alpha-synuclein and TOM20 preserved these mitochondrial proteins.
The level of mitochondrial protein GRP75, which is imported by TOM20, is known to increase in response to mitochondrial stress. While GRP75 production was not significantly affected by alpha-synuclein overexpression, the combined production of alpha-synuclein and TOM20 led to a robust fivefold increase in GRP75 in dopaminergic neurons.
“These data suggest that GRP75 imparts an important protective function in mitochondria damaged by [alpha]-synuclein accumulation,” the researchers wrote.
“Collectively, these data indicate that TOM20 expression prevents [alpha]-synuclein-induced mitochondrial dysfunction, which is sufficient to rescue dopaminergic neurons in the adult rat brain,” the researchers wrote.
“As such, the [alpha]-synuclein–TOM20 interaction may represent an important target for therapeutic intervention,” they concluded.






