New Biosensor May Shed Light on Molecular Processes Involved in Parkinson’s
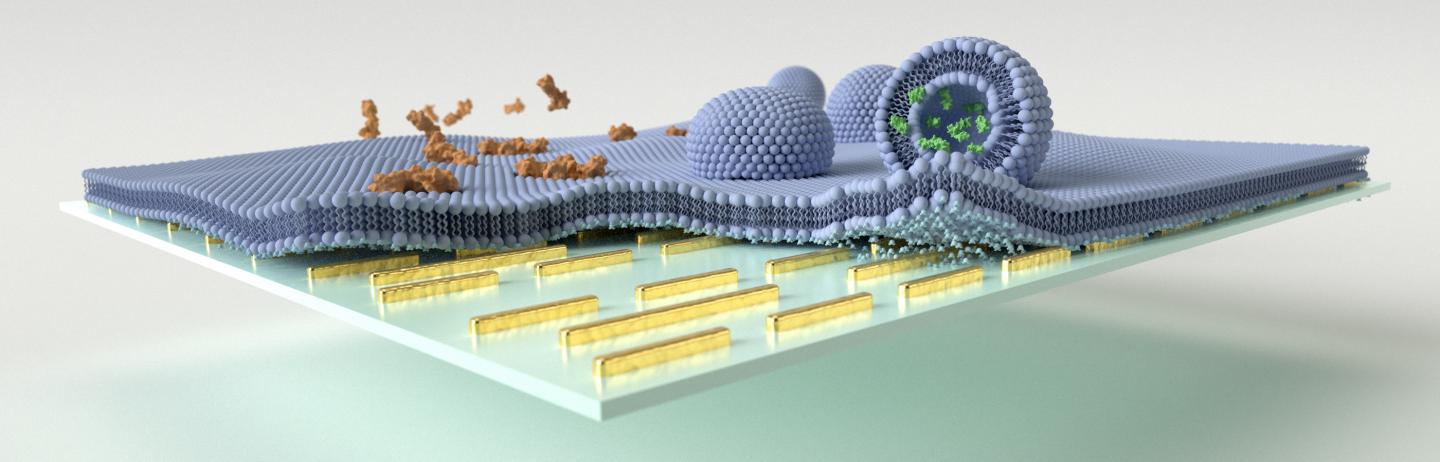
Multi-resonant mid-IR nanoantennas are leveraged to enhance the vibrational absorption signals associated with biomimetic lipid membrane formation, polypeptide/membrane interaction, and vesicular cargo release on the sensor surface. Credit: EPFL
New sensors with increased sensitivity and specificity to detect interactions between proteins, fat molecules, and other biological elements, represent a powerful tool to investigate mechanisms linked to human diseases, such as Alzheimer’s or Parkinson’s.
Those findings were highlighted in the study “Resolving molecule-specific information in dynamic lipid membrane processes with multi-resonant infrared metasurfaces,” which was published in Nature Communications.
The way biomolecules react and interact with each other is crucial to sustain normal cellular function, and to ensure all cellular mechanisms are properly working. This comprises mechanisms such as molecular signaling and transport in cells that require insertion of proteins with the cell lipid membrane.
Until now, available label-free techniques were not able to differentiate simple cellular processes such as protein insertion, chemical release and membrane disruption. As such, researchers had to rely on multiple experimental methods to analyze and understand the processes taking place within cells.
This not only made the experimental setting more complex, it also made it more difficult to integrate all data into single response models.
To overcome these challenges, a team led by researchers at Ecole Polytechnique Fédérale de Lausanne in Switzerland developed a new biosensor system.
The mid-infrared biosensor has the capacity to access the distinct chemical fingerprint information of proteins, lipids, or other biochemical compounds in complex biological samples while monitoring their dynamic interactions.
A major advantage of this new technology is that it allows scientists to preform complex analysis without destroying samples, in real-time, and with high sensitivity.
The team tested the potential of the biosensor to evaluate the molecular changes of cell membranes and tiny vesicles loaded neurotransmitter molecules (responsible for communication between brain nerve cells) upon exposure to melittin, the major toxic component of the honeybee venom.
The biosensor allowed researchers to monitor the disruptive mechanisms triggered by melittin in real-time and without requiring any additional process of molecule labeling.
The team could track the processes by which melittin induced membrane disruption and neurotransmitter cargo release from the vesicles.
“Our sensor opens up exciting possibilities for gaining new insights into biological processes such as signaling or transport in basic research as well as provides a valuable toolkit for bioanalytical and pharmaceutical applications,” researchers wrote.
These findings pave the way to apply this biosensor to several research fields, and investigate important mechanisms linked to human diseases, such as pore formation and membrane disruption induced by protein aggregates in neurodegenerative diseases.






