3-D ‘Scaffolds’ Supporting Healthy Neurons Offer New Stem Cell-Treatment Approach for Parkinson’s
Written by |
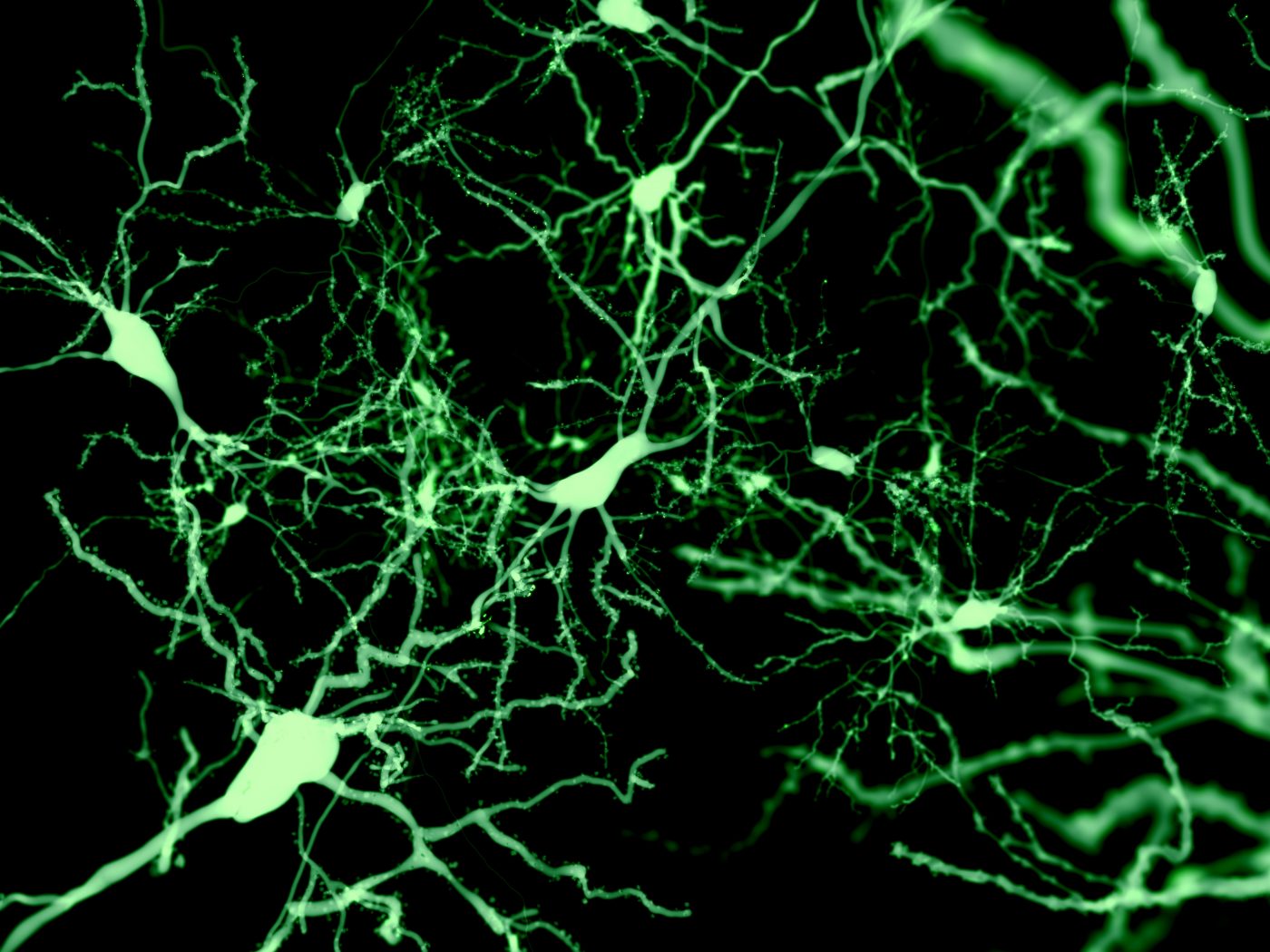
Rutgers and Stanford University researchers have developed 3-D “scaffolds,” or fibers, that can support healthy and high-functioning human neurons derived from adult stem cells, which can be transplanted to the brain to replace diseased neurons. The technology represents a possible new therapeutic strategy for numerous neurological conditions, including Parkinson’s and Alzheimer’s disease, amyotrophic lateral sclerosis, and multiple sclerosis.
The study detailing the platform, “Generation and transplantation of reprogrammed human neurons in the brain using 3D microtopographic scaffolds,” was published in Nature Communications.
Parkinson’s disease results from the loss of dopamine-producing nerve cells, causing impaired movement, balance, and coordination. Cell replacement therapy with human pluripotent stem cell-derived neurons has been explored as a potential therapy for neurodegenerative dysfunction and central nervous system injury. Efforts have largely failed, however, as reprogrammed neurons are dissociated and disorganized during transplantation, resulting in poor cell survival and functionality in vivo.
Researchers designed a 3-D scaffold of micro polymer fibers, which promotes in situ stem cell neuronal reprogramming and neuronal engraftment in the brain. When the scaffolds were injected into the brains of mice, researchers reported a 100-fold increase in neuronal survival over other methods used in transplantation.
“If you can transplant cells in a way that mimics how these cells are already configured in the brain, then you’re one step closer to getting the brain to communicate with the cells that you’re now transplanting,” Professor Prabhas V. Moghe, the study’s senior author and research director for the School of Engineering/Health Sciences Partnerships at Rutgers, said in a press release. “In this work, we’ve done that by providing cues for neurons to rapidly network in 3-D.”
Future research plans include the improvement of scaffold biomaterials to increase the number of implanted neurons in the brain, allowing for greater therapeutic benefits. The idea is to create a network of high functioning and better controlled neurons. Testing in mouse models of Parkinson’s disease is currently underway, but researchers estimate the technology will need another 10 to 20 years of work to be ready for clinical trials.


