Deep Brain Stimulation Sounds Like Science Fiction But Can Quiet Parkinson’s Tremors

The 27th annual Supercomputing Conference (SC15), formally known as The International Conference for High Performance Computing, Networking, Storage and Analysis, will be held in the Austin Convention Center at Austin, Texas from November 15-20 2015, with an attendant exhibition to be open from November 16-19.
The conference, sponsored by ACM (Association for Computing Machinery) and IEEE Computer Society will offer a complete technical education program and exhibition to showcase the many ways high performance computing, networking, storage and analysis lead to advances in scientific discovery, research, education and commerce. This premier international conference includes a globally attended technical program, workshops, tutorials, a world class exhibit area, demonstrations and opportunities for hands-on learning. For more information on SC15, visit: https://www.sc15.supercomputing.org/
The latest release in a video series originally launched by the 2013 SC conference steering committee highlights innovative research being done at the University of Utah’s Scientific Computing and Imaging Institute focused on helping Parkinson’s Disease (PD) patients lead more normal lives through deep brain stimulation (DBS).
“Deep Brain Stimulation” sounds a bit ominous, perhaps evoking science fiction, but new surgery techniques that place a set of wires under the skull to transmit electrical signals to different areas of the brain, while not new in concept, has become even more effective with the power of computers applied and enhanced using high performance computing and visualization. However, the fact that DBS can quiet tremors associated with Parkinson’s Disease and other brain disorders, the prospect of which should also calm any patient apprehension.
The Brain Initiative for Advancing Innovative Neurotechnologies (BRAIN), announced in 2013, opens opportunities to unlock mysteries of the brain and accelerate the development of research and technologies to treat disorders such as Parkinson’s Disease. For more information about the BRAIN initiative, see: https://www.nih.gov/science/brain.
The National Institute of Neurological Disorders and Stroke (NINDS), a branch of the National institutes of Health (NIH), supports DBS research on determining the technique’s safety, reliability, and effectiveness as a PD treatment. NINDS support for research on brain circuitry has been critical to development of DBS.
Deep Brain Stimulation can be used to treat several disabling neurological symptoms — most commonly debilitating motor symptoms associated with Parkinson’s disease such as tremors, rigidity, stiffness, slowed movement, and problems with walking. The procedure is also used to treat essential tremor and dystonia.
 A video featuring Dr. Melissa Houser, clinical director of the Parkinsons Disease and Movement Disorder Center at Scripps Clinic in San Diego, leading a discussion about DBS can be found here. Topics covered include how DBS works, who is a candidate for the procedure, expected outcomes, and personal commentary from three DBS-implanted patients.
A video featuring Dr. Melissa Houser, clinical director of the Parkinsons Disease and Movement Disorder Center at Scripps Clinic in San Diego, leading a discussion about DBS can be found here. Topics covered include how DBS works, who is a candidate for the procedure, expected outcomes, and personal commentary from three DBS-implanted patients.
According to the National Parkinson Foundation, DBS does not damage healthy brain tissue by destroying nerve cells. Instead, the procedure blocks electrical signals from targeted areas in the brain.
At present, DBS, which was FDA-approved in 1997, is used only for patients whose symptoms cannot be adequately controlled with medications. However, only individuals who improve to some degree after taking medication for Parkinson’s benefit from DBS. A variety of conditions may mimic PD but do not respond to medications or DBS, which uses a surgically implanted, battery-operated medical device called an implantable pulse generator (IPG) — similar to heart pacemakers and approximately the size of a stopwatch to — deliver electrical stimulation to specific areas in the brain that control movement, thus blocking the abnormal nerve signals that cause PD symptoms associated with movement disorders, most commonly Parkinson’s, but also essential tremor, and dystonia.
Prior to the procedure, a neurosurgeon uses magnetic resonance imaging (MRI) or computed tomography (CT) scanning to identify and locate the exact surgical intervention target within the brain where electrical nerve signals generate the PD symptoms. Some surgeons may also use microelectrode recording — which involves a small wire that monitors activity of nerve cells in the target area — to more specifically identify the precise brain area that will be stimulated. There are three brain targets that have been FDA approved for use in Parkinson’s disease: the thalamus, subthalamic nucleus (STN), and globus pallidus interna (GPi). There is a low chance that placement of the stimulator may cause bleeding or infection in the brain. Ongoing studies that will help to refine target choice for individual patients.
 The DBS system’s three main components include the lead, the extension, and the IPG. The lead (also called an electrode) is a thin, insulated wire inserted through a small opening in the skull and implanted in the brain. The electrode’s tip is positioned within the specific brain area.
The DBS system’s three main components include the lead, the extension, and the IPG. The lead (also called an electrode) is a thin, insulated wire inserted through a small opening in the skull and implanted in the brain. The electrode’s tip is positioned within the specific brain area.
The extension is an insulated wire that is passed under the skin of the patient’s head, neck, and shoulder, connecting the lead to the implantable pulse generator. The IPG (AKA “battery pack”) is usually implanted under the skin near the collarbone, but in some cases may be implanted lower in the chest or under the skin of the abdomen.
Once its components are in place, electrical impulses are transmitted from the IPG through the extension wire and the lead and into the brain. These impulses block abnormal electrical signals and alleviate PD motor symptoms.
Unlike other types of surgery for PD, DBS involves minimal permanent surgical changes to the brain, but rather electrical stimulation to regulate electrical signals in neural circuits to and from identified areas in the brain to improve PD symptoms. The less-invasiv,e permanent nature of DBS means that if there is a manifestation of unwanted side effects or should newer, more promising treatments be developed in the future, the implantable pulse generator can be removed, and the DBS procedure halted. Another advantage of DBS is that IPG stimulation intensity is easily adjustable without further surgery should the patient’s condition change.
Even after undergoing DBS, most individuals will continue needing medication, but according to NINDS, many people with Parkinson’s Disease experience considerable relief from their motor symptoms and are able to reduce medication levels. The amount of reduction varies but can be substantial within most individuals, permitting a significant improvement in drug side effects such as dyskinesias (involuntary movements caused by long-term use of levodopa). In some cases, the stimulation itself can suppress dyskinesias without a reduction in medication. However, a downside is that DBS does not improve cognitive symptoms in PD and indeed may worsen them, so the technique is not generally used on patients presenting with signs of dementia. And while DBS changes the brain’s firing pattern, it does not slow progression of neurodegeneration.
DBS research continues to find ways to improving the technology. A two-part study funded by NINDS and the Department of Veterans Affairs first compared bilateral DBS to best medical therapy, including medication adjustment and physical therapy. Bilateral DBS showed overall superiority to best medical therapy at improving motor symptoms and quality of life.
The second part of the study, involving nearly 300 patients, compared subthalamic nucleus (STN) DBS to globus pallidus interna (GPI) DBS. The two groups reported similar improvements in motor control and quality of life in scores on the Unified Parkinson’s Disease Rating Scale. On a variety of neuropsychological tests, there were no significant differences between the two groups. However, the STN DBS group experienced a greater decline on a test of visuomotor processing speed, which measures how quickly someone thinks and acts on information. Also, the STN DBS group had slight worsening on a standard assessment of depression, while the GPI DBS group had slight improvement on the same test. The importance of these two differences is not clear, and will be scrutinized in follow-up research.
In addition, NINDS-supported researchers are developing and testing improved implantable pulse generators, and conducting studies to better understand the therapeutic effect of neurostimulation on neural circuitry and brain regions affected in PD. For more information about current studies on brain stimulation and Parkinsons disease, see https://www.clinicaltrials.gov and search for deep brain stimulation AND Parkinson AND NINDS. For information about NINDS-and NIH-supported research studies in this area, see the NIH RePORTER (Research Portfolio Online Reporting Tools) at https://projectreporter.nih.gov and search for deep brain stimulation AND Parkinson.
 “We know DBS does not change the progression of Parkinson’s disease, but it does improve people’s quality of life after they have it,” says Paul House, MD, a neurosurgeon and the surgical director of University of Utah Health Care’s Movement Disorders Program who himself has done more than 400 of the surgeries at University of Utah Health Care (UUHC). According to Dr. House, they get a lot more years of improved quality of life. Nationally, UUHC ranks in the top 15 for number of annual DBS procedures with some of the best patient outcomes.
“We know DBS does not change the progression of Parkinson’s disease, but it does improve people’s quality of life after they have it,” says Paul House, MD, a neurosurgeon and the surgical director of University of Utah Health Care’s Movement Disorders Program who himself has done more than 400 of the surgeries at University of Utah Health Care (UUHC). According to Dr. House, they get a lot more years of improved quality of life. Nationally, UUHC ranks in the top 15 for number of annual DBS procedures with some of the best patient outcomes.
 Recently the program hired Christopher Butson, PhD, as its director of neuromodulation research. An expert in neurostimulation devices and neuromodulation therapy, Dr. Butson came to Utah from the Medical College of Wisconsin’s Biotechnology and Bioengineering Center at Milwaukee. One of Dr. Butson’s goals at UUHC is more use of neurostimulation therapy to improve the lives of patients with a range of neurological and psychiatric disorders.
Recently the program hired Christopher Butson, PhD, as its director of neuromodulation research. An expert in neurostimulation devices and neuromodulation therapy, Dr. Butson came to Utah from the Medical College of Wisconsin’s Biotechnology and Bioengineering Center at Milwaukee. One of Dr. Butson’s goals at UUHC is more use of neurostimulation therapy to improve the lives of patients with a range of neurological and psychiatric disorders.
Another key component in Dr. Butson’s work: data. UUHC has an extensive high performance computing infrastructure that is the raw material for much of his work. Many of those rich details have not been — but now will be — mined for data relevant to new discovery and innovation. “The one thing we aspire to do in my lab is to combine computational models with patient data to create insights that would be difficult to achieve otherwise,” Dr. Butson notes.
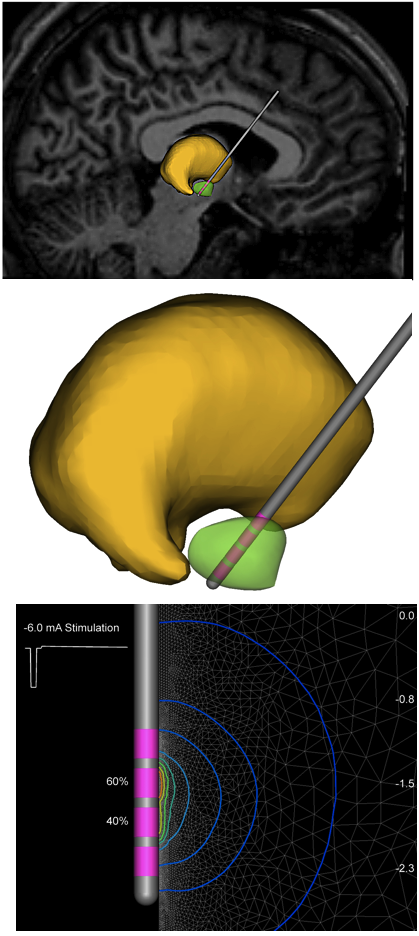
One of Dr. Butson’s most promising ideas is an Apple iPad-based interactive computer program that creates a three-dimensional image of the brain. This provides clinicians with a more comprehensive look at a patient’s brain and can help them make better determination regarding DBS lead placements, stimulation settings and effects as well as saving time. Clinicians who used the program in a small pilot study reduced device programming time by a whopping 99 percent. The program, which allows for simulated stimulation of the brain’s circuits, may eventually be expanded to help enhance precision of surgical targets and DBS treatment for a multitude of disorders and diseases.
 Dr. Butson and Co-PI Dr. Michael Okun at the University of Florida were recently awarded a grant from the National Institutes of Health to conduct a clinical trial of the iPad based system in a much larger patient cohort. It’s an innovation that until recently wasn’t possible, but has been made so by advances in medical imaging technology – another area of research that UUHC helped advance.
Dr. Butson and Co-PI Dr. Michael Okun at the University of Florida were recently awarded a grant from the National Institutes of Health to conduct a clinical trial of the iPad based system in a much larger patient cohort. It’s an innovation that until recently wasn’t possible, but has been made so by advances in medical imaging technology – another area of research that UUHC helped advance.
“Where the field is going is toward circuit-based therapy… and we hope that 3D modeling will allow us to see this in a totally different way,” Dr. Butson says. “So integrating the best information we can get from all different types of imaging and incorporating that into predictive models… this is the bleeding edge of DBS research.”
To see the brief two minute video go to:
https://youtu.be/qx2dRIQXnbs
Sources:
University of Utah Scientific Computing and Imaging (SCI) Institute
SC15
Vitals.com
Image Credits:
University of Utah Scientific Computing and Imaging (SCI) Institute
Scripps Clinic
University of Florida Health






