Neurofeedback System Lets Patients Control Brainwaves Linked to Movement, Researchers Say
Written by |
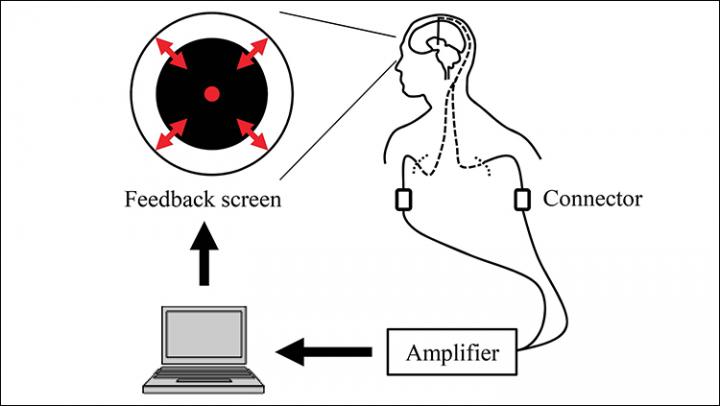
Feedback system overview. Signals from the DBS electrodes were acquired in real time. The radius of the black circle on the computer screen was controlled based on the β-band power of the acquired bipolar signals from adjacent contacts that were selected in the pre-feedback session.
Parkinson’s disease patients voluntarily controlled brainwaves associated with motor symptoms using a neurofeedback system, according to new research.
These findings have implications for how to best harness brain activity and deep brain stimulation to treat Parkinson’s disease symptoms.
The study, “Real-time neurofeedback to modulate β-band power in the subthalamic nucleus in Parkinson’s disease patients,” was published in eNeuro.
Many studies have reported a relationship between the beta-band power — a brainwave with a frequency between 12 and 30Hz — in the subthalamic nucleus and motor symptoms in Parkinson’s disease. The subthalamic nucleus is a brain region thought to implement the so-called “hyperdirect pathway” of motor control critical to suppressing erroneous movement.
Parkinson’s disease is characterized by abnormal neuronal oscillations in the subthalamic nucleus. Consequently, abnormal oscillations of beta-band power are commonly observed and correlate with disease symptoms.
The use of dopaminergic (L-dopa) medications has been shown to decrease beta-band oscillations while relieving Parkinson’s disease symptoms, such as bradykinesia — impaired ability to move one’s body.
Similarly, researchers have been able to suppress beta-band oscillation in the subthalamic nucleus using deep brain stimulation (DBS) — a neurosurgical procedure involving the implantation of a device that electrically activates specific clusters of neurons in the brain to treat movement disorders.
However, the best way to use deep brain stimulation to treat Parkinson’s disease symptoms remains unclear.
There are two main types of deep brain stimulation currently being examined for therapeutic use: continuous DBS and adaptive DBS. Continuous deep brain stimulation applies the same electrical stimulation continuously without any external control, the way a pacemaker controls a constant heartbeat; adaptive deep brain stimulation changes the amount of electrical stimulation with the fluctuating beta-band oscillations.
Recent studies have demonstrated that adaptive deep brain stimulation using beta-band oscillation reduced Parkinson’s disease symptoms more effectively than continuous DBS. These improvements correlated with attenuation of beta-band oscillations. As such, “beta-band oscillation in the [subthalamic nucleus] may be a therapeutic target for clinical interventions such as rehabilitation,” researchers stated.
However, it was unclear whether Parkinson’s patients could voluntarily moderate beta-band oscillations in the subthalamic nucleus, which could change the way deep brain stimulation is implemented to improve Parkinson’s rehabilitation.
For these reasons, researchers at Osaka University in Japan wanted to test the relationship between brain activity and disease symptoms in Parkinson’s patients. They examined whether Parkinson’s patients could voluntarily or involuntarily influence control over adaptive deep brain stimulation- generated impulses using a neurofeedback system — sensors that monitor how fast or slow one’s brainwaves are firing and subconsciously teaches the brain to fire at optimal speeds.
To do so, the researchers studied brain activity from deep brain stimulation electrodes implanted in the subthalamic nucleus in eight patients who were connected to a neurofeedback system.
The researchers examined subthalamic nucleus activity before and after a 10-minute test. Patients were shown a circle whose diameter was controlled by their own beta-band power through the neurofeedback system. The patients were instructed to make the radius of a black circle on a computer screen smaller by using their thoughts and without moving their bodies. If the size of the circle changed throughout the test, it would mean that the patients were voluntarily influencing their beta-band power.
Four patients were induced to decrease the beta-band power during the feedback training (down-training condition); the others were induced to increase (up-training condition).
For the four patients in the down-training group, beta-band power was significantly decreased after the training, whereas only two out of four patients in the up-training group showed a significant increase in the beta-band power. “The powers in other frequency bands … did not, however, change significantly before and after the neurofeedback training,” researchers noted.
Overall, the patients could control the beta-band power in the subthalamic nucleus through neurofeedback. However, the researchers did not observe reductions in patients’ motor symptoms.
These results show that feedback training successfully demonstrated that the beta-band power of the subthalamic nucleus could be modulated to increase or decrease based on patients’ voluntary control.
The neurofeedback training may be an effective method for revealing the functional changes that accompany atypical beta-oscillations. Although this method did not relieve Parkinson’s disease symptoms, the study paves the way for a new approach toward managing Parkinson’s-related brain activity that could inform the development of new treatments.
“Here, we have developed a novel neurofeedback technique using intracranial electrodes implanted in deep brain structures to modulate subthalamic nucleus activity,” researchers said. “This is the first report to demonstrate that human patients with Parkinson’s disease were able to voluntarily control their [beta]-band power in subthalamic nucleus to induce changes in the power.”