Mutation Common to Familial Parkinson’s and Tuberculosis May Lead to Ways of Treating Both Diseases
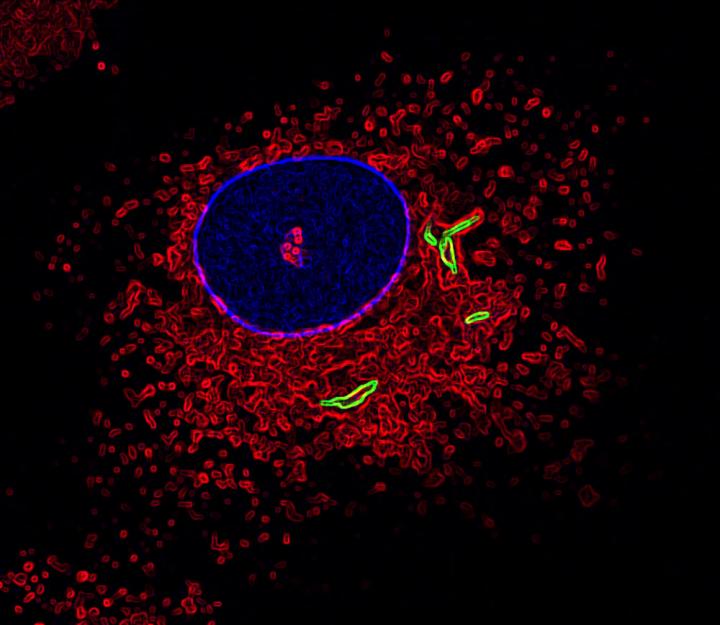
This is an image of a macrophage labeled for the nucleus (blue), fused lysosomes and phagosomes (red) and TB bacteria (green). (Photo by Susanne Herbst, Francis Crick Institute)
A mutation common to familial Parkinson’s was seen in a study focused on tuberculosis to prevent immune cells of the brain from working properly, allowing protein to accumulate in nerve cells — and, potentially, to this specific disease’s development.
The study, “LRRK2 is a negative regulator of Mycobacterium tuberculosis phagosome maturation in macrophages,” appeared in The EMBO Journal.
Mutations in the LRRK2 gene are the most common genetic cause of Parkinson’s disease. As these alterations result in excessive activation of the protein LRRK2 kinase, researchers are focused on developing treatments that might block LRRK2. But exactly how LRRK2 causes Parkinson’s and why its blockers work is not well understood. (About 15 percent of Parkinson’s patients have a family history of the disease and identifiable mutations.)
Besides Parkinson’s, mutations in the LRRK2 gene are associated with chronic inflammation and bacterial infections. Again, however, the gene’s precise role in immunity is unclear.
When bacteria, or any unwanted microorganism, enters the body, immune cells called macrophages kick into action. Macrophages are responsible for recognizing and engulfing the bacteria in small compartments called phagosomes. Another cellular structure, called the lysosome, then fuses with the phagosome to destroy the bacteria trapped inside the phagosomes.
Using a combination of experimental strategies, researchers found that LRRK2 prevented phagosomes and lysosomes from fusing as they should in both human and mouse macrophage cells infected with Mycobacterium tuberculosis (Mtb) — the pathogenic bacteria that causes tuberculosis. Importantly, treating these cells with an LRRK2 blocker significantly reduced Mtb levels.
Mice genetically modified to lack the LRRK2 protein had an quicker immune response against Mtb, and lower levels of this bacteria in their lungs up to two weeks after infection compared to normal mice.
“Importantly, these data uncover LRRK2 as a regulator of the early clearance of Mtb,” the researchers wrote.
“We think that this mechanism might also be at play in Parkinson’s disease, where abnormal masses of protein called ‘Lewy bodies’ build up in neurons in the brain and cause damage,” Susanne Herbst, PhD, with The Francis Crick Institute and the study’s co-first author, said in a press release.
LRRK2 may prevent immune cells in the brain from properly breaking down debris, leading to a protein build-up in neurons that disrupts their function.
“By studying TB, we have found a possible explanation for why LRRK2 mutations are a genetic risk factor for Parkinson’s,” Herbst said.
Patrick Lewis, a study’s co-author and a professor at the University of Reading, noted that while research in Parkinson’s has focused on neurons and why they degenerate, the potential key role of other cells, particularly those involved in the immune response, is increasingly appreciated.
“This study reinforces why we should think more broadly about the events that cause neurodegeneration, and that some of the answers to Parkinson’s disease might come from immunology,” Lewis said.
The scientists’ work may also lead to new TB treatments, taken from work in Parkinson’s. “LRRK2 inhibiting drugs are already being developed to treat Parkinson’s disease and we’re trying to see if we can repurpose them as a potential new TB therapy,” said Max Gutierrez, the study’s senior author. “This should be relatively straightforward because TB infects the lungs, so the LRRK2 inhibitors wouldn’t need to cross the blood-brain barrier like they do in Parkinson’s disease.”






