Genetic Risk of Parkinson’s Disease Increased by Low Selenium Intake, Study Shows
Written by |
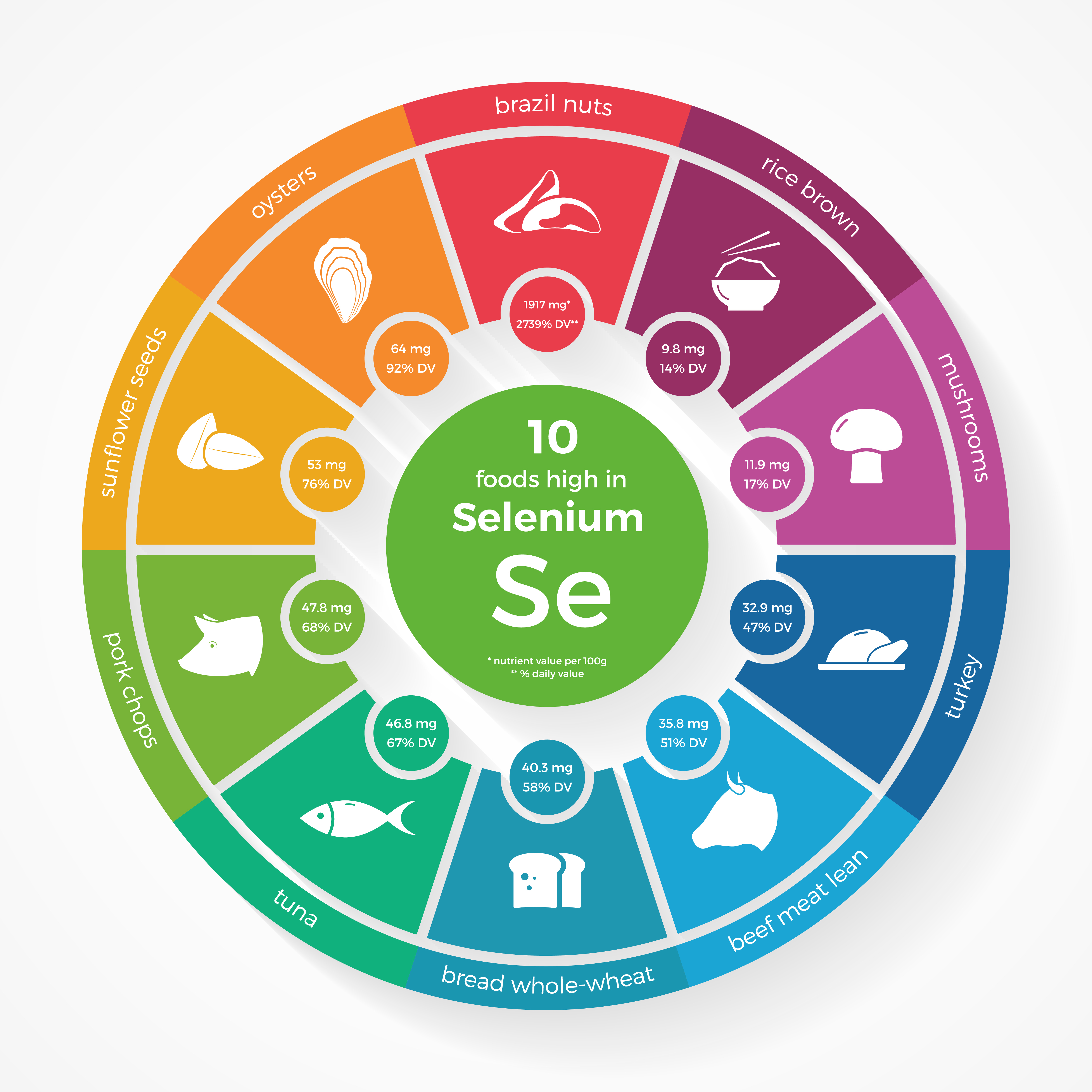
Low selenium intake may increase the risk of developing Parkinson’s disease in people carrying a particular genetic mutation, according to a study on environmental and genetic interactions and their impact on Parkinson’s disease (PD) development.
The research demonstrated that cataloging knowledge of gene-environment interactions is indispensable for leveraging knowledge from epidemiological and genetic studies, and correctly assessing the impact of specific genes.
The study, “High-throughput allele-specific expression across 250 environmental conditions,” was published in the journal Genome Research.
Interactions between environmental factors and genes are extremely complex to assess in living organisms, particularly in humans. So, to make reaching conclusions easier, researchers at Wayne State University School of Medicine‘s Center for Molecular Medicine and Genetics in Michigan used cultured cells.
The research team exposed the cells to more than 250 environmental factors, including caffeine and common drugs. Then they studied how the cells’ genes reacted to the treatment.
“Our cellular system simplifies the complexity of the environment,” Francesca Luca, PhD, assistant professor of molecular medicine and genetics, and one of the co-senior authors of the study, said in a press release.
The effort led to an identification of 215 genes that reacted to the environmental changes. Half of them are genes that are crucial for human traits or diseases. In addition to the finding that low selenium intake may expose people carrying the LAMP3 gene to an increased risk of Parkinson’s disease, the team also found that caffeine intake may decrease the risk to develop obesity in people carrying a protective gene.
“Both genes and environmental conditions are major influences on our health and who we are,” said Luca. “For example, stress is a risk factor for cardiovascular disease; however, the actual risk to have a heart attack depends not only on the amount of stress in a person’s life but also on the specific DNA sequences — genetic variants — that he or she inherited from their parents,” she said.
“The interplay between genetic variants and environments during human evolutionary history provided the driving force that shaped our genome. Today, genetic adaptations that helped us in the past to better store energy in fat, for example, can make us more likely to develop a disease,” Luca added.
The research team is now exploring molecular mechanisms that may explain how the identified environmental factors, such as selenium, impact the effect of a gene. They also are searching for more interactions, and performing similar studies in a large number of individuals of African-American origin to assess other genetic variants and their interactions.


