Neurons Grown in 3-D From Stem Cells Could Lead To Parkinson’s Disease Breakthrough

Progressive loss of neurons in the brains of Parkinson’s patients is a slow yet inexorable process, and so far, there are no drugs that can halt this insidious process. However, teams of researchers at the Luxembourg Centre for Systems Biomedicine (LCSB) of the University of Luxembourg have now succeeded in growing the types of neurons affected by Parkinson’s starting from neuronal stem cells in a three-dimensional cell culture system.
The team of scientists working with Dr. Ronan Fleming of the LCSB research group Systems Biochemistry Group are confident that the system they’ve developed could significantly advance the continuing search for therapeutic agents in the future, since it models natural conditions in the brain more realistically than other systems currently available, and is also significantly cheaper to employ in the laboratory.
The Systems Biochemistry Group, is an interdiscipinary research group of mathematical, computational and experimental biologists whose mission and fundamental interest is to conduct research on systems of biochemical reactions in general and to develop scalable mathematical and numerical analysis techniques to be applied in particular to aetiopathogenesis and amelioration of Parkinson’s disease.
 Photo Caption: The Systems Biochemistry group at the LCSB – Top row (from left to right): Siham Hachi, Edinson Lucumi, Dr. Ronan Fleming, Miguel Oliveira, Diana El Assal. Bottom row (from left to right): Dr. Hulda Haraldsdottir, Dr. Vuong Phan, Dr. Longfei Mao, Fatima Monteiro
Photo Caption: The Systems Biochemistry group at the LCSB – Top row (from left to right): Siham Hachi, Edinson Lucumi, Dr. Ronan Fleming, Miguel Oliveira, Diana El Assal. Bottom row (from left to right): Dr. Hulda Haraldsdottir, Dr. Vuong Phan, Dr. Longfei Mao, Fatima Monteiro
Systems biochemistry is the application of a systems approach to understand biochemistry. A systems approach consists of an iterative cycle of mathematical and computational modelling and quantiative experimental measurement. Mathematical and computational models are formal representations of biochemical knowledge that are used to propose hypotheses, design experiments and interpret experimental results. Quantitative experimental measurements are used to test hypotheses generated by the model and generate data used to refine the model. Model predictions are used for optimal experimental design and compared with quantitative experimental data, including that obtained from their microfluidic cell cultures of dopaminergic neurons, derived from normal and Parkinsonian human subjects using stem cell biology techniques.
The LCSB researchers’ findings were recently published in the journal Lab on a Chip in a paper entitled “Paper Differentiation of neuroepithelial stem cells into functional dopaminergic neurons in 3D microfluidic cell culture” (Lab Chip, 2015,15, 2419-2428, DOI: 10.1039/C5LC00180C), co-authored by Edinson Lucumi Moreno, Siham Hachi, Kathrin Hemmer, Aidos S. Baumuratov, Jens C. Schwamborn, and Ronan M. T. Fleming of the Luxembourg Centre for Systems Biomedicine at the University of Luxembourg; and Sebastiaan J. Trietsch, Thomas Hankemeier, and Paul Vulto of Mimetas B.V, and the Analytical BioSciences Division of the Leiden Academic Centre for Drug Research at Leiden University — both in Leiden, The Netherlands.
The researchers note that progressive loss of nigrostriatal dopaminergic neurons is a hallmark of Parkinson’s disease. They report success in deriving human neuroepithelial cells from induced pluripotent stem cells and successfully differentiating them into dopaminergic neurons within phase-guided, three-dimensional microfluidic cell culture bioreactors. They note that after 30 days of differentiation in these microfluidic bioreactors, in situ morphological, immunocytochemical and calcium imaging confirmed the presence of dopaminergic neurons that were spontaneously electrophysiologically active, a characteristic feature of nigrostriatal dopaminergic neurons in vivo.
The coauthors report that differentiation was as efficient as in macroscopic culture, with up to 19 percent of differentiated neurons immunoreactive for tyrosine hydroxylase, the penultimate enzyme involved in dopamine synthesis, and conclude that this new microfluidic cell culture model integrates the latest innovations in developmental biology and microfluidic cell culture to generate a biologically realistic and economically efficient route to personalized drug discovery for Parkinson’s disease.
The LCSB is dedicated to accelerating biomedical research by strengthening the link between systems biology and medical research, with collaboration among biologists, medical doctors, computer scientists, physicists and mathematicians offering new insights in complex systems like cells, organs, and organisms that are essential for understanding principal mechanisms of disease pathogenesis and for developing new tools in diagnostics and therapy.
Neurodegenerative diseases like Parkinson’s disease, metabolomics and disease network analysis are in the focus of LCSB’s research. Within Luxembourg, the LCSB is building up strong partnerships with all major biological and medical research units. Key strategic partnership has been set-up with the Institute for Systems Biology
(ISB) in Seattle, Washington — a cooperation that is enabling a rapid scientific evolution. The LCSB fosters collaboration with industrial partners and will be a focal point for developing a knowledge based economy in Luxembourg.
Parkinsons disease (PD) is the second most common neurodegenerative disease an is typically characterized by its four motor symptoms: resting tremor, slow movement, walking difficulty and postural instability. However PD is also associated with many non-motor symptoms such as depression and loss of olfactory sense that will typically occur before the disease is diagnosed. PD is further characterized by presence of abnormal neuronal masses, known as Lewy Bodies, in those neurons that remain.
Parkinson’s currently affects over 1.2 million people across Europe with close to 1200 confirmed PD cases in tiny Luxembourg alone. The annual cost of PD is estimated to be 13.9 billion euros while the number of people with PD is set to double by 2030 with an increasing ageing population (https://www.epda.eu.com).
The Clinical and Experimental Neuroscience group at the LCSB headed by Prof. Dr. Rejko Kruger aims to create an essential bridge between basic research and the clinic so that effective prevention, diagnosis and therapy may help the patients in Luxembourg and beyond.
 Photo Caption: The Clinical & Experimental Neuroscience group at the LCSB. From left to right: Rejko Kruger, Caroline Obermaier, Yvonne Eberling, Dajana Grossmann, Mary Faltz, Ibrahim Boussaad, Peter Barbuti
Photo Caption: The Clinical & Experimental Neuroscience group at the LCSB. From left to right: Rejko Kruger, Caroline Obermaier, Yvonne Eberling, Dajana Grossmann, Mary Faltz, Ibrahim Boussaad, Peter Barbuti
Prof. Dr. Kruger also does weekly consultations at the Centre Hospitalier de Luxembourg (CHL) where he applies his long-standing Parkinsons disease expertise for the benefit the patients by giving individual advice for treatment options. Patients as well as healthy people can also directly contribute to PD research by donating biological samples (e.g. DNA, skin). DNA samples can be screened genetically for new PD genes, while cells can be grown from skin samples to be used to detect distinctive patterns for disease. The researchers note that interestingly, from skin cells they are able to check for disease patterns similar to those found in the neurons.
Results generated from the group’s basic research in collaboration with other groups at the LCSB will help to identify new biomarkers and therapeutic targets. Using automated drug discovery approaches coupled with advanced imaging methods, they can then identify new treatments for PD, not only in the form of medication but also alternative methods such as deep brain stimulation. This comprehensive strategy facilitates successful translation of pre-clinical discoveries back to the patients including advanced diagnostics and treatments for PD.
Parkinson’s disease is characterized in particular by the death of dopamine-producing neurons in the Substantia nigra of the midbrain. It is already possible to grow these dopaminergic neurons in cell cultures, “But most such cell cultures are two-dimension
al, with the cells growing along the base of a petri dish, for example,” group leader and Lab Chip paper corresponding author Dr. Ronan Fleming explains. “Instead, we have the neurons grow in a gel that yields a far better model of their natural, three-dimensional environment.”
As a starting point for cultivating the target neurons, the scientists use ordinary skin cells which they convert using conventional methods into induced pluripotent stem cells, or iPSCs for short. For the development of this technology Japanese scientist Shinya Yamanaka was awarded the Nobel Prize for Physiology or Medicine in 2012 together with British scientist John B. Gurdon. “By adding suitable growth factors, the iPSCs
can then be converted in a second step into neural stem cells,” says Prof. Jens Schwamborn, Lab Chip paper coauthor and head of the LCSB research group Developmental & Cellular Biology, which is responsible for the differentiation of the cells, in a release. “These are the starting cells we use in the microfluidic culture.”
The researchers first mix the cells with a liquid, which they then fill into little test vessels called bioreactors. “You can imagine such a bioreactor as a tunnel separated down the middle by a flat
barrier,” LCSB researcher Edinson Lucumi Moreno, first author of the Lab Chip study, explains. “One side of the tunnel we load the liquid with the cells, where it hardens into a gel under controlled temperatures. The other side we load with a medium to which we can add nutrients and substances for further differentiation of the neuronal stem cells as required.”
After only a few hours, the researchers can already observe changes in the neuronal stem cells, which begin to form little protrusions that develop over the following days into axons and dendrites — the long extensions typical of neurons. After 30 days, 91 percent of the cells are neurons, about 20 percent of which are the desired dopaminergic neurons — a result the scientists say has been confirmed in morphological and immunological tests.
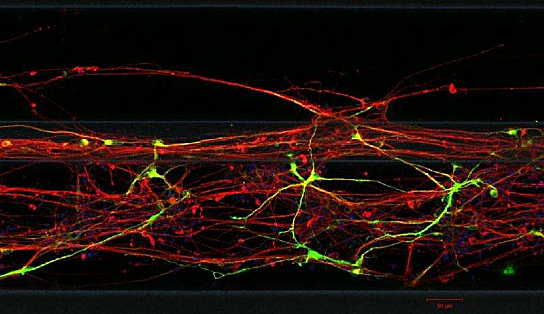
Neurons Grown From Skin Cells – Photo Credit (c) LCSB 2015
One of the major advantages of this 3D cell culture system is that it can already be automated in its present form: The bioreactors are placed on commercially available plates that can be processed and read out by laboratory robots. “In drug development, dozens of chemical substances can therefore be tested for possible therapeutic effects in a single step,” says Dr. Fleming. “Because we use far smaller amounts of substances than in conventional cell culture systems, the costs drop to about one tenth the usual.”
Another advantage is that the bioreactors can be loaded with cells originating from the skin cells of individual Parkinson’s patients. “This is an important step towards personalized drug development,” Dr. Fleming observes. As a next step, Dr. Fleming’s team and their international collaborators want to study cells from patients and to test potential active pharmaceutical ingredients. Promising candidates among which will then be tested in mice.
Sources:
University of Luxembourg
Luxembourg Centre for Systems Biomedicine
Lab Chip
Image Credits:
Sources:
University of Luxembourg






