Cell therapy ANPD001 safe for first 2 trial Parkinson’s patient groups
ASPIRO is testing therapy's safety, tolerability over one year
Written by |
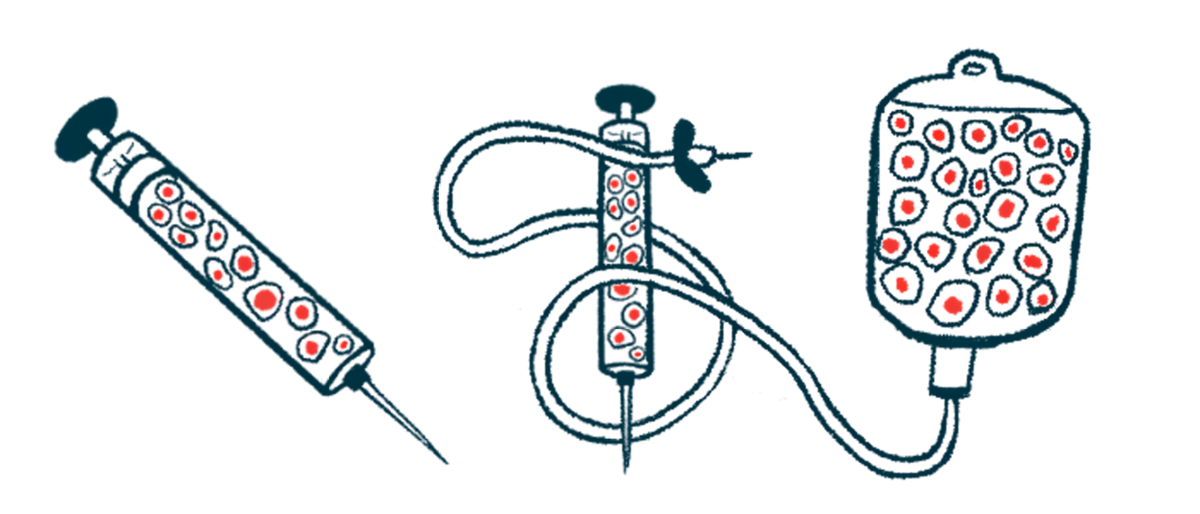
Aspen Neuroscience has completed dosing the first two groups of patients in ASPIRO, an ongoing Phase 1/2a clinical trial that’s testing the safety and tolerability of ANPD001, an investigational cell therapy for Parkinson’s disease.
Patients with moderate to severe Parkinson’s who have so far received ANPD001, which uses a patient’s own cells injected into the brain under general anesthesia, have had no serious side effects. All were sent home within 48 hours as planned.
“To date, ANPD001 and its delivery have been well tolerated, and no serious adverse events have been observed,” Edward Wirth III, MD, PhD, Aspen’s chief medical officer, said in a company press release. “All patients were discharged within 48 hours, per protocol.”
Testing ANPD001
Launched last year, the open-label ASPIRO trial (NCT06344026) is testing how safe and well tolerated ANPD001 is over one year when injected at escalating doses in patients, ages 50-70. Researchers will also watch for changes in on time, when motor symptoms are under control, over a year.
“This is a major milestone for the first multi-patient, multicenter clinical trial of an autologous therapy for Parkinson’s disease,” Wirth said. “We are now advancing the program to investigate our new commercial formulation,” one that may be prepared at scale for the market, if ANPD001 is approved in the future.
Motor symptoms of Parkinson’s occur due to the loss of dopaminergic neurons, the nerve cells that produce dopamine in the brain. Dopamine is a signaling chemical that acts on brain regions responsible for motor control. Less dopamine results in slowed movements, tremor, stiffness, and problems with balance.
ANPD001 is designed to replace the lost dopaminergic neurons with new ones. Because ANPD001 uses a patient’s own cells, no immunosuppressants are needed to stop the immune system from rejecting the cell therapy. This makes it safer and more personalized.
The first step is to collect a sample of a patient’s skin cells, which are converted into stem cells and then given chemical cues to develop into precursors of dopaminergic neurons. They are infused back into the patient by MRI-guided injection into a brain region called the putamen, where they’re expected to mature into fully functional dopaminergic neurons.
ANPD001 has received fast track designation from the U.S. Food and Drug Administration, which may help speed up its development and review. Fast track benefits include frequent guidance, faster approval processes, and eligibility for priority review and accelerated approval.