Xadago Launched in US as Add-on Treatment for Parkinson’s Disease
Written by |
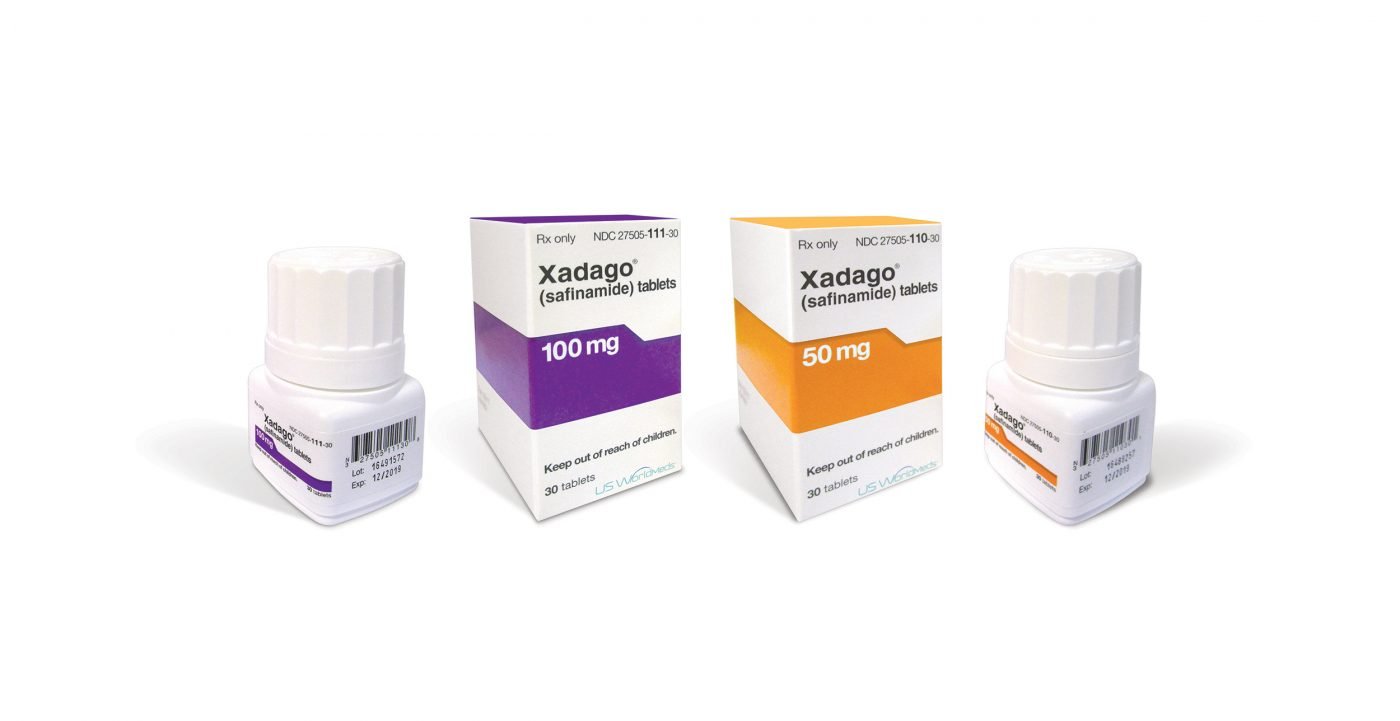
Xadago (safinamide) is now available in the U.S. as an add-on treatment for patients with Parkinson’s disease currently taking levodopa/carbidopa to control their “off” episodes, according to an announcement from Newron Pharmaceuticals and US WordMeds.
Xadago is the first medication approved in more than 10 years by the U.S. Food and Drug Administration (FDA) to manage motor deficits in Parkinson’s patients. “Off” periods are marked by an increase in disease symptoms, such as tremor and difficulties in walking, when a drug’s effects are wearing off or aren’t working well.
“The approval of Xadago offers an important new treatment option for the Parkinson’s community,” Stuart Isaacson, MD, director of the Boca Raton Institute for Neurodegenerative Disorders, said in a news release.
“Xadago is the first New Chemical Entity approved for the treatment of [Parkinson’s disease]-related motor fluctuations in the U.S. in over a decade. In clinical trials, patients on once-daily Xadago demonstrated significant improvement in ‘on’ time without troublesome dyskinesia.”
The approval of Xadago was supported by positive results obtained in clinical trials with more than 1,100 patients. These studies demonstrated that patients on levodopa treated with Xadago experienced significant increases in “on” time (when the drug works properly and symptoms are reduced) without dyskinesia (uncontrolled involuntary movement), and reduced periods of “off” time.
“We are proud to see Xadago become the first FDA-approved new chemical entity for Parkinson’s disease in more than 10 years,” said Stefan Weber, CEO of Newron Pharmaceuticals, based in Milan, Italy. “We believe Xadago has the potential to significantly improve the quality of life of [Parkinson’s disease] patients in the U.S., as it already has in many other countries around the world.”
P. Breckinridge Jones, CEO of US WorldMeds, based in Louisville, Kentucky, said his company is excited to be launching the new drug in the U.S.
“Part of US WorldMeds’ mission is to develop and market meaningful and accessible healthcare products that improve lives and result in a thriving community of patients,” Jones said. “We are confident that Xadago will progress that mission by providing a new treatment option to Parkinson’s patients.”
The drug selectively inhibits MAO-B, a form of the protein MAO that breaks up several neurotransmitters, including dopamine. A lack of dopamine is linked to the development of Parkinson’s disease. By inhibiting dopamine degradation in the brain, MAO-B inhibitors help control motor symptoms, which are associated with reduced levels of dopamine.
These types of drugs can either be used as a monotherapy or an add-on treatment to improve the action of other drugs, such as levodopa, a dopamine precursor.