Lipid Plays Key Role in Nerve Cell Death in Parkinson’s Disease, Study Suggests
Cardiolipin, a molecule inside nerve cells, may be a key player behind nerve cell death in Parkinson’s disease, a study suggests.
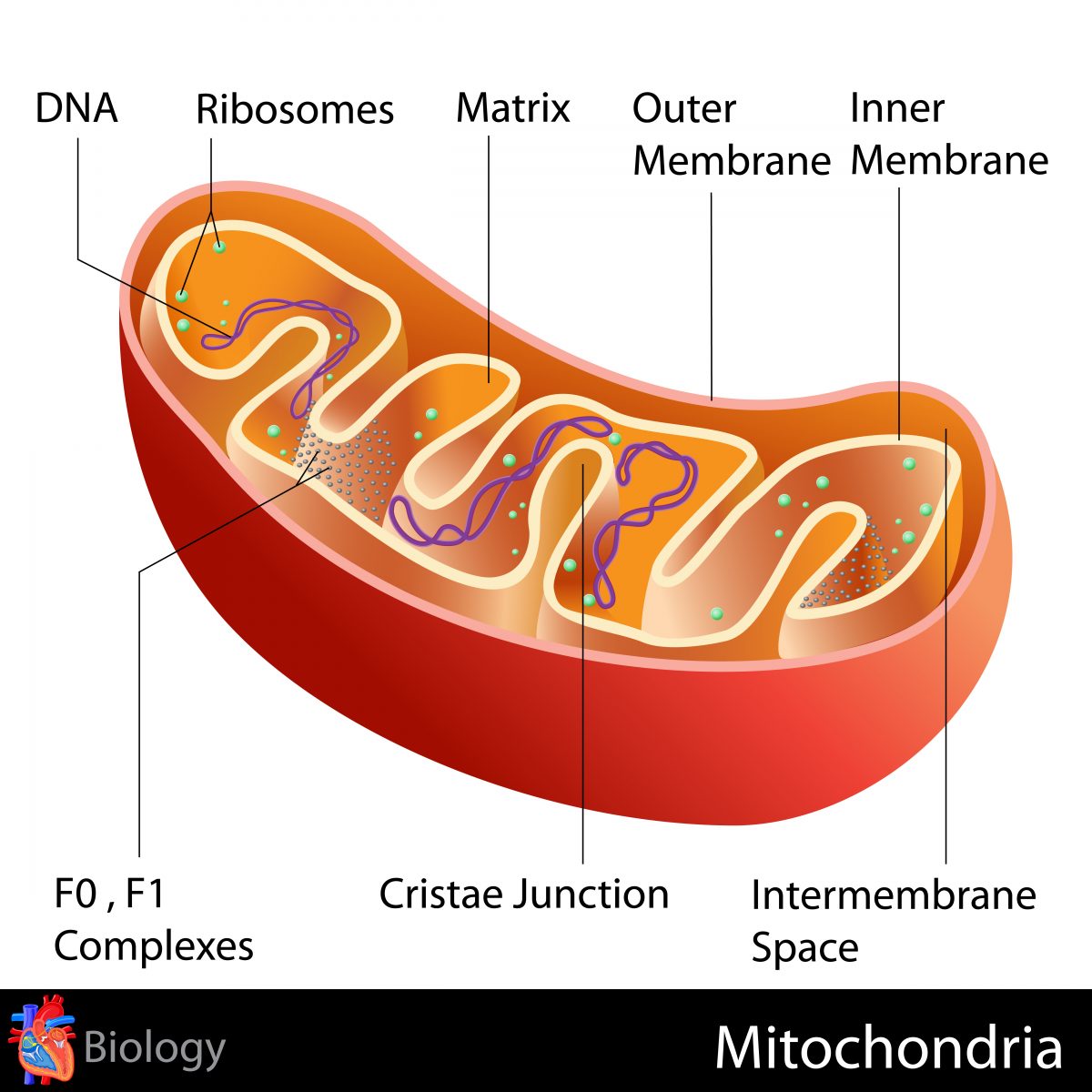
Researchers found that cardiolipin, a lipid inside mitochondria — the cell’s energy source — ensures the correct 3-D arrangement (folding) of the alpha-synuclein protein.
Misfolding of this protein leads to deposits in the brain that are the hallmark of Parkinson’s disease.
University of Guelph researchers in Canada have now determined how impaired mitochondria are linked to abnormal protein folding.
The study, “Cardiolipin exposure on the outer mitochondrial membrane modulates α-synuclein,” was recently published in the journal Nature Communications.
“Identifying the crucial role cardiolipin plays in keeping these proteins functional means cardiolipin may represent a new target for development of therapies against Parkinson’s disease,” Scott Ryan, PhD, the study’s lead author, said in a press release. “Currently there are no treatments that stop nerve cells from dying.”
The team used stem cells from Parkinson’s patients to investigate how nerve cells deal with abnormally folded alpha-synuclein.
“We thought if we can better understand how cells normally fold alpha-synuclein, we may be able to exploit that process to dissolve these aggregates and slow the spread of the disease,” said Ryan, a professor in the Department of Molecular and Cellular Biology at University of Guelph.
They found that cardiolipin, a lipid that sits in the inner membrane of mitochondria, plays a vital role in the folding of alpha-synuclein protein. The lipid moves to the outer membrane of the mitochondria where it binds the misfolded alpha-synuclein and helps the protein refold so it doesn’t accumulate in toxic clumps.
In cells carrying alpha-synuclein mutations linked to familial Parkinson’s disease, researchers saw a prolonged exposure of cardiolipin on the outer surface of mitochondria, which eventually triggers mitochondria destruction in nerve cells.
“As a result, the cells slowly die. Based on this finding, we now have a better understanding of why nerve cells die in Parkinson’s disease and how we might be able to intervene,” Ryan said.
Understanding the role of cardiolipin in Parkinson’s disease may help researchers develop new therapies for the disease.
“The hope is that we will be able to rescue locomotor deficits in an animal model. It’s a big step towards treating the cause of this disease,” he concluded.






