Great Lakes NeuroTech Allowed Patent Claims for Parkinson’s Deep Brain Stimulation Visualization Maps
Valley View, Ohio-based Great Lakes NeuroTechnologies (GLNT) have announced receipt of a new allowance of claims from the U.S. Patent Office focused on technology for Parkinson’s disease (PD) diagnostics and treatment.
This particular patent application covers a method of measuring Parkinson’s motor symptoms such as tremor, bradykinesia, and rigidity, and creating visualization maps to assist with programming deep brain stimulation (DBS), a neurosurgical procedure involving implantation of a medical device called a neurostimulator. That device sends electrical impulses, through implanted electrodes, to specific parts of the brain for treatment of movement and affective disorders, then adjusting stimulation settings to a level that alleviates symptoms without causing side effects.
Specifically, the allowed claims acknowledge the unique and proprietary nature of using objective, wearable sensors to measure symptom response to DBS programming, and to create visual programming maps displaying objective data that clinicians can use to manually adjust stimulation settings. In addition, the application of automated programming algorithms to search these maps to determine optimal settings is highly valuable.
GLNT notes that while DBS has been shown effective for treating Parkinson’s motor symptoms, there can be disparity in outcomes among implanted patients due to varied post-operative management, particularly concerning DBS programming optimization. Programming DBS settings for Parkinson’s disease can be challenging for clinicians, and the company’s Kinesia technology provides automated tools for quantification of tremor, assessment of dyskinesia, and measuring bradykinesia, all of which can assist with DBS programming. GLNT’s Kinesia 360 device provides a telemedicine solution that allows clinicians to screen patients before DBS surgery and follow patient symptoms at home after programming.
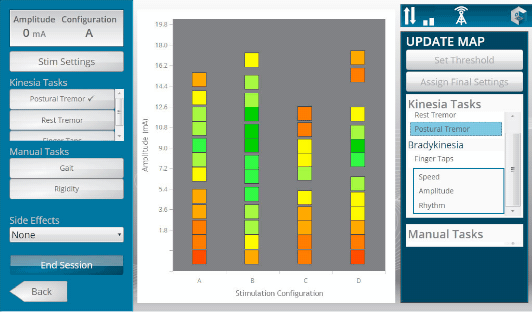
GLNT previously launched Kinesia ProView for creating DBS visualization maps. During a programming session, the patient wears a sensor while data is sent wirelessly to a mobile platform with an app to process objective symptom scores and display them as a function of DBS settings.
Kinesia ProView technology provides tuning maps during clinic programming sessions to help visualize symptom response to DBS settings. Both systems utilize broadband integrated mobile platforms with patient worn sensors, and the Kinesia web application to define clinical protocols and review reports.
Programmers can adjust several types of settings, including the contact, polarity, frequency, pulse width, and amplitude of stimulation. This provides for a tremendous array of possible combinations that must be evaluated over multiple types of motor symptoms in a limited time frame during an office visit.
To address this problem, the ProView system provides a standardized platform to quantitatively assess how symptoms such as tremor, bradykinesia, and dyskinesias change in response to specific DBS settings. The system integrates a secure, HIPAA-compliant web application and a broadband-enabled tablet interface with wireless patient sensors.
During a programming assessment, the patient wears a wireless sensor to assess motor symptoms at each DBS setting. A tablet PC application creates color-coded tuning maps as the programming session progresses, which display specific symptom severities as a function of stimulation settings. This tuning map allows physicians to visually assess the programming parameter space and optimize the final settings for specific symptoms and battery consumption. Once the programming session is complete, patient data and tuning maps are transmitted via broadband connectivity to a cloud-based server for storage, reporting, and trending using the Kinesia web application.
GLNT commercialized its proprietary Kinesia technology to provide wearable, objective and automated assessment of movement disorder symptoms. That technology is now being deployed in targeted applications such as advanced therapy screening and titration for Parkinson’s disease.
 “Advanced therapies for Parkinson’s, such as deep brain stimulation, can greatly improve quality of life for patients. However, adjusting the settings on a DBS system to maximize therapeutic benefit within a short programming session can be challenging. This is especially true as, more DBS systems receive regulatory approvals with increased options for contacts and stimulation patterns,” says Joseph P. Giuffrida, PhD, President and Principal Investigator. “Intuitive maps that allow visual representation of symptom response as a function of stimulation settings can provide a unique interface to guide the programming session, improving both the clinician and patient experience.”
“Advanced therapies for Parkinson’s, such as deep brain stimulation, can greatly improve quality of life for patients. However, adjusting the settings on a DBS system to maximize therapeutic benefit within a short programming session can be challenging. This is especially true as, more DBS systems receive regulatory approvals with increased options for contacts and stimulation patterns,” says Joseph P. Giuffrida, PhD, President and Principal Investigator. “Intuitive maps that allow visual representation of symptom response as a function of stimulation settings can provide a unique interface to guide the programming session, improving both the clinician and patient experience.”
Parkinson’s disease is the second most common neurodegenerative disease after Alzheimer’s in the U.S. Although promising research is being conducted, there is currently no cure for Parkinson’s disease, a slowly progressing neurodegenerative disorder caused by the death of dopaminergic neurons in a region of the midbrain called the substantia nigra for reasons unknown. Dopamine is a neurotransmitter that carries signals between the areas of the brain that regulate and control smooth, purposeful motor movement when performing routine tasks like eating, writing, and shaving.
Common first symptoms of Parkinson’s include tremors, rigidity and slowness of movement. While the motor symptoms can be treated with medication, the disease’s progression cannot be prevented, and the benefits of medication may fade as the disease progresses and/or side effects can become problematic. People with late stage Parkinson’s disease have many disabling symptoms, including trouble walking, impaired posture and balance, muscle stiffness, and tremors in the arms and hands that make it difficult to perform everyday tasks.
In addition, Parkinson’s disease may cause non-motor symptoms such as sleep problems, depression, and anxiety, which unfortunately are not alleviated by current Parkinson drugs. An estimated 7 million people worldwide are living with Parkinson’s disease, and the National Institutes of Health estimates that about 1 million Americans have Parkinson’s disease, with some 50,000 Americans being diagnosed with new Parkinson’s cases annually.
For more information, visit:
https://www.glneurotech.com
Sources:
Great Lakes NeuroTechnologies
National Institutes of Health