First Patient Treated in Study Testing Aleva’s directSTIM DBS System
Written by |
Aleva Neurotherapeutic’s innovative directional deep brain stimulation (DBS) system — called directSTIM — for Parkinson’s disease has been implanted in the first patient participating in a German study.
That study, which aims to demonstrate improved outcomes when using this DBS technology in individuals with Parkinson’s and essential tremor, will enroll up to 60 patients across Germany.
The first surgical procedure was conducted at the University Hospital of Dresden by neurosurgeon Stephan Sobottka, MD, MBA.
The final results of this European post-market clinical follow-up (PMCF) study are expected in the first half of 2022. A PMCF study is aimed at keeping up-to-date the security and effectiveness of a medical device approved in the EU.
“Enrolling a first patient in our PMCF study is a significant milestone in Aleva´s development,” André Mercanzini, PhD, Aleva’s CEO, said in a press release.
“Our Deep Brain Stimulation System incorporates several technologies that will provide better outcomes for Parkinson’s patients,” Mercanzini said.
Aleva plans to submit an investigational device exemption (IDE) application to the U.S. Food and Drug Administration by the end of this year. If approved, the IDE will allow directSTIM to be used in a clinical study with the goal of collecting safety and efficacy data.
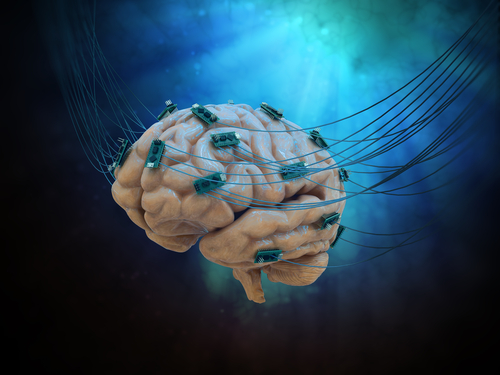
Deep brain stimulation is a surgical procedure that involves placing electrodes in specific areas of the brain to deliver electrical impulses generated by a battery-operated neurostimulator. It is used for the treatment of Parkinson’s motor symptoms.
Contrary to other DBS systems in which the electrical impulses are fired in all directions — a system called omnidirectional — the directSTIM uses electrodes able to promote a directional release of the electrical signals. This makes the DBS more precise and efficient, and reduces the risk for potential side effects.
Moreover, Aleva’s directSTIM uses smaller, and round-shaped electrodes — contrary to rectangular electrodes in other DBS systems — which have been shown to be safe and allow for enhanced directional fields.
In a pilot study with Parkinson’s patients, the directSTIM DBS was applied to two brain regions involved in motor control, the subthalamic nucleus and the nucleus ventralis intermedius. It was found to improve clinical efficacy by reaching the expected beneficial effects at a lower current, and also increasing the current threshold at which side effects appear.
Based on technology developed at the Swiss Federal Institute of Technology (EPFL), Lausanne, the directSTIM has 24 independent current sources and 12 directional electrodes. This allows more flexibility for the surgical team, according to Aleva.
“The directSTIM system is simple and straightforward to use,” Sobottka said. “We will be able to immediately determine the advantages of directional stimulation, and we look forward to completing the study with Aleva.”
The system is based on its proprietary microengineered leads, which use MEMS or microelectromechanical systems technology.
“As one of the originators of Directional Deep Brain Stimulation, Aleva has done a substantial amount to study this modality and its benefit to patients,” said Andres Lozano, a professor with the department of neurosurgery at the University of Toronto, and a senior medical advisor to Aleva.
“We expect to quantitatively determine its specific advantages within the Aleva study,” Lozano added.