Stable Parkinson’s Disease Drug Therapy Status Quo Could Soon Be Disrupted by New Category Entrants
Written by |

Burlington, Massachusetts-based, medical market research firm Decision Resources Group (DRG) reports, citing analyses of U.S. longitudinal patient-level claims data, that the Parkinson’s disease (PD) drug treatment algorithm remains highly stable, with levodopa (either alone or in combination with carbidopa) as its foundation.
Parkinson’s disease (PD) is a chronic, progressive, neurological disorder with a poorly understood cause, in which the nerve cells that produce the natural brain chemical dopamine are damaged and unable to produce enough of the biochemical agent. The resultant diminished dopamine levels cause a variety of symptoms and problems associated with movement, including tremors (shaking), stiffness, and slowness of movement. The primary Parkinson’s drug levodopa (L-dopa) belongs to a class of medications called central nervous system agents, and works by being converted to dopamine in the brain.
Levodopa’s sometime companion PD drug carbidopa is from a medication class called decarboxylase inhibitors, and works by preventing levodopa from breaking down before it reaches the brain, thus allowing lower doses of levodopa, resulting in fewer unpleasant side effects like nausea and vomiting, according to the National Institutes of Health.
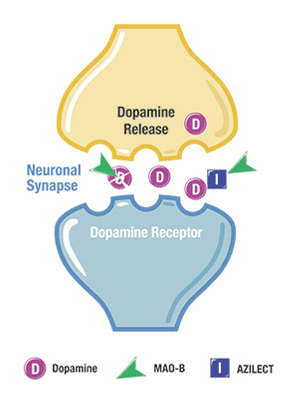 DRG notes that among newly diagnosed patients, Teva’s Azilect (rasagiline tablets) is now the second most frequently prescribed Parkinson’s drug after levodopa, claiming a 10 percent to 12 percent patient share in the first line over a five-quarter study period ending December 31, 2014. Azilect is designed to regulate the activity of an enzyme in the brain, called MAO-B, that breaks down dopamine, making it unavailable for the brain to use, thus causing an even greater decline in dopamine levels in people with Parkinson’s. Azilect is an MAO-B inhibitor — ergo, a medication that blocks MAO-B, thereby allowing more dopamine to be available for use in the brain, helping preserve both a patient’s own dopamine and dopamine derived from levodopa therapy.
DRG notes that among newly diagnosed patients, Teva’s Azilect (rasagiline tablets) is now the second most frequently prescribed Parkinson’s drug after levodopa, claiming a 10 percent to 12 percent patient share in the first line over a five-quarter study period ending December 31, 2014. Azilect is designed to regulate the activity of an enzyme in the brain, called MAO-B, that breaks down dopamine, making it unavailable for the brain to use, thus causing an even greater decline in dopamine levels in people with Parkinson’s. Azilect is an MAO-B inhibitor — ergo, a medication that blocks MAO-B, thereby allowing more dopamine to be available for use in the brain, helping preserve both a patient’s own dopamine and dopamine derived from levodopa therapy.
DRG finds the observed consistency in PD therapeutics understandable given recent absence of major PD-indicated drug launches. However, they report that a range of novel therapies are poised to meaningfully impact PD treatment decisions in the near future.
Insights from Decision Resources Group’s Parkinson’s Disease research include:
Improving on Levodopa Is a Key Development Goal
As the “gold standard” of PD drug treatment, levodopa captured a nearly 70 percent patient share among recently treated PD patients in Q4 2014, according to U.S. claims data. DRG observes that a diverse range of late-stage levodopa reformulations from Intec Pharma, Neuroderm, and others are aiming to exploit the clinical shortcomings of levodopa and thereby tap into a large potential patient pool.
In February 2015, Impax Laboratories launched in the United States its product Rytary — a combined immediate and controlled release formulation of levodopa with carbidopa. With its three-times-daily dosing and benefits in reducing “off” time, DRG’s analysts in their 10-year major-market forecast expect Rytary to achieve notable uptake, largely for patients with intermediate to advanced PD.
Rescue Alternatives Are Needed
Phenomena known as “off episodes” are considered one of the most frustrating and challenging complications of Parkinson’s disease progression. These flare-ups can make muscle stiffness, slow movements, and difficulty starting movements very difficult, and interfere with a patient’s ability to walk, stand, and even speak.
According to pharma firm WorldMed, incidence of off episodes is relatively high in PD patients with a history of long-term oral levodopa use, increasing with the length of time they are on levodopa medication.
Today, DRG says patients requiring immediate relief from “off” episodes have one option: injectable apomorphine — a strong dopamine agonist, that is: drugs that simulate the actions of dopamine in the brain. Apomorphine injections, e.g., US WorldMeds’ Apokyn, are used in addition to oral PD medicines as needed to treat off-episode loss of control of body movements in people with advanced Parkinsons disease.
However, DRG analysts note that fewer than 1 percent of recently treated patients used the costly drug in Q4 2014. Interviewed experts are enthusiastic that Acorda’s CVT-301 — a self-administered, inhaled levodopa therapy for episodic treatment of off episodes in Parkinson’s disease — and Cynapsus’ lead product candidate, APL-130277 — a sublingual thin film formulation of apomorphine — will present effective, patient-friendly alternatives that could meaningfully increase the number of patients who seek and receive a rescue option. DRG forecasts strong sales potential in this arena.
Parkinson’s Disease Psychosis Is Prevalent and Underserved
A safe and effective treatment for PD psychosis remains an unmet need for roughly one-third of PD patients, according to DRG epidemiological estimates. DRG’s analysts observe that approximately 10 percent of recently treated patients received off-label treatment with an atypical antipsychotic in Q4 2014, but current options have modest efficacy, a limited evidence base, and bear safety risks. However, they suggest that Acadia’s Nuplazid — a selective serotonin inverse agonist (SSIA) preferentially targeting 5-HT2A receptors — could become the first therapy approved specifically for PD psychosis, and following projected U.S. and European launches in 2016, they forecast the drug will start to unlock this market opportunity, garnering peak-year sales above $500 million in PD treatment.
 Decision Resources Group analyst Tamara Blutstein, PhD comments:
Decision Resources Group analyst Tamara Blutstein, PhD comments:
“Until recently, the PD pipeline was stagnant, but new drugs have the potential to bring real therapeutic advances in key areas of opportunity. The result, according to our latest PD forecast, is a greater than $1 billion increase in the PD drug market in the G7 by 2024.
“With an abundance of effective, generic options, a major challenge for novel entrants in the PD drug market will be successfully navigating the market access landscape, particularly in Europe. Recent DRG research with EU5 payers makes clear that a high bar is set for new PD brands; demonstrating clinically meaningful improvements over appropriate comparators — in efficacy, above all — will be the surest path to positive [Health Technology Assessment (HTA)] outcomes.”
For more information about Decision Resources Group, visit:
https://DecisionResourcesGroup.com
Sources:
Decision Resources Group
Parkinson’s UK
National Institutes of Health U.S. National Library of Medicine
US Worldmeds, LLC
Impax Laboratories
Accorda Therapeutics
Cynapsus
Acadia Pharmaceuticals Inc.