Nanobodies Could Prevent Alpha-Synuclein’s Toxic Effects in Parkinson’s, Rat Study Suggests
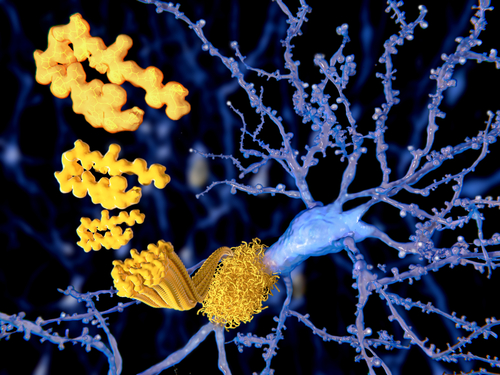
Antibody fragments, known as nanobodies, may be an efficient strategy to reduce abnormal alpha-synuclein protein aggregates and preserve motor function in Parkinson’s disease and other neurodegenerative disorders, a rat study suggests.
The study, “Proteasome-targeted nanobodies alleviate pathology and functional decline in an α-synuclein-based Parkinson’s disease model,” was published in the journal npj Parkinson’s Disease.
Parkinson’s disease belongs to a class of neurodegenerative disorders called synucleinopathies, characterized by the accumulation of misfolded alpha-synuclein aggregates. These abnormal protein aggregates are toxic to brain cells, triggering several damaging processes that promote the impairment and death of cells.
Given the key role of alpha-synuclein in the development and progression of Parkinson’s disease, many efforts have been made to find ways to effectively prevent its toxicity.
A team led by researchers at Rush University Medical Center in Chicago have developed a new strategy to target alpha-synuclein using nanobodies, small fragments of antibodies that can target and bind to specific proteins, tagging them for destruction.
In a previous study, the team showed that two particular nanobodies, called VH14 (also known as NAC14) and NbSyn87, could affect alpha-synuclein’s ability to aggregate. In the current study, they explored these nanobodies’ potential to prevent the progression of Parkinson’s in rats.
The team used an adenovirus-based system to deliver each nanobody directly into the substantia nigra — an area of the brain highly affected in Parkinson’s — of rats with Parkinson’s disease. This treatment was selectively administered to one side of the brain, or hemisphere, but not the other. An adenovirus is an inactivated virus that is very stable and easy to produce and can act as a “shell” to transport molecules inside cells. They are widely used for gene therapy purposes.
Treating the rats with either VH14 or NbSyn87 led to a nearly twofold reduction of the relative amount of toxic protein aggregates, compared with rats treated with a placebo. Notably, the nanobodies did not promote excessive destruction of alpha-synuclein, allowing residual protein activity to be maintained and preventing total loss of function.
Further detailed analysis revealed that rats treated with VH14 had 49% increased innervation in the striatum, compared with placebo-treated animals, and a 38% increase, compared with NbSyn87-treated rats. The striatum is a brain region involved in motor and reward systems, which receives dopamine inputs from different sources.
In addition, VH14 showed greater potential to protect the nerve cells in the substantia nigra from damage, whereas NbSyn87 showed just modest neuroprotective potential.
Evaluation of dopamine levels in the striatum region failed to show significant improvements upon treatment with either nanobody. Still, when comparing the treated versus non-treated brain hemispheres, animals treated with VH14 had about three times higher levels of dopamine, compared with placebo-treated rats.
Despite the variable impact on dopamine output, researchers reported that animals treated with VH14 had significant improvements in motor function over placebo-treated rats, restoring their walking ability and balance. Treatment with NbSyn87 also appeared to have a positive impact on preserving the animals’ motor function, but at a slower rate than VH14.
According to the researchers, these results demonstrate the “therapeutic efficacy of vector-delivered intracellular nanobodies targeting alpha-synuclein misfolding and aggregation in synucleinopathies such as Parkinson’s disease.”