New Treatment Strategy Slows Progression of Parkinson’s Disease in Mice
 Researchers at Johns Hopkins University School of Medicine report their discovery that a protein called LAG3 acts as an enabler for a toxic natural aggregate to spread from cell to cell in the brains of mammals causing Parkinson’s disease development, and also of a means of blocking that protein’s action.
Researchers at Johns Hopkins University School of Medicine report their discovery that a protein called LAG3 acts as an enabler for a toxic natural aggregate to spread from cell to cell in the brains of mammals causing Parkinson’s disease development, and also of a means of blocking that protein’s action.
In a research article published in the journal Science, the Johns Hopkins investigative team describes key findings of their study in mice and cultured cells that suggest an immunotheapy currently in clinical trials as a cancer therapy should also be tested as a way to slow the progression of Parkinson’s disease (PD).
The article, Pathological-synuclein transmission initiated by binding lymphocyte-activation gene 3, notes that emerging evidence indicates that the development of Parkinson’s disease may be due to cell-to-cell transmission of misfolded preformed fibrils (PFF) of -synuclein (-syn), although the mechanism by which -syn PFF spreads from neuron to neuron is not yet known.
In their article, the researchers show that the lymphocyte-activation gene 3 (LAG3) binds -syn PFF with high affinity, while the -syn monomer showed minimal binding, and -syn-biotin PFF binding to LAG3 initiated -syn PFF endocytosis, transmission, and toxicity.
They conclude that the lack of LAG3 substantially delayed -syn PFF induced loss of dopamine neurons, as well as biochemical and behavioral deficits in mice. As a result, identifying LAG3 as a receptor that binds -syn PFF creates a target for developing new therapies designed to slow Parkinson’s progression of and related conditions.
 One of the study’s lead authors, Dr. Ted Dawson, MD, PhD, a professor of neurology and director of the Institute for Cell Engineering at Johns Hopkins School of Medicine, explains in a press release that these new findings are predicated on how alpha-synuclein protein aggregates enter brain cells.
One of the study’s lead authors, Dr. Ted Dawson, MD, PhD, a professor of neurology and director of the Institute for Cell Engineering at Johns Hopkins School of Medicine, explains in a press release that these new findings are predicated on how alpha-synuclein protein aggregates enter brain cells.
He noted that abnormal clumps of alpha-synuclein protein are frequently found in autopsies of Parkinson’s disease patients and are thought to cause dopamine-producing brain cell death.
Dawson cites evidence for a novel theory that Parkinson’s disease progresses as alpha-synuclein aggregates spread from one brain cell to another. The theory, published by a researcher at Goethe University in Germany, suggests these migrations induce previously normal alpha-synuclein proteins to aggregate, gradually moving from lower brain structures regulating movement and basic functions to higher areas associated with functions like memory and reasoning. Dawson notes that the German scientist’s theory was greeted with skepticism, but then other labs began showing that alpha-synuclein might spread from cell to cell.
 Intrigued, Dawson’s research group began working with findings from Johns Hopkins
Intrigued, Dawson’s research group began working with findings from Johns Hopkins  professor of neurology Valina L. Dawson, PhD, director of the neuroregeneration and stem cell programs at the Johns Hopkins Medicine Institute for Cell Engineering, and assistant professor of neurology Han Seok Ko, PhD, to investigate how the aggregates enter cells.
professor of neurology Valina L. Dawson, PhD, director of the neuroregeneration and stem cell programs at the Johns Hopkins Medicine Institute for Cell Engineering, and assistant professor of neurology Han Seok Ko, PhD, to investigate how the aggregates enter cells.
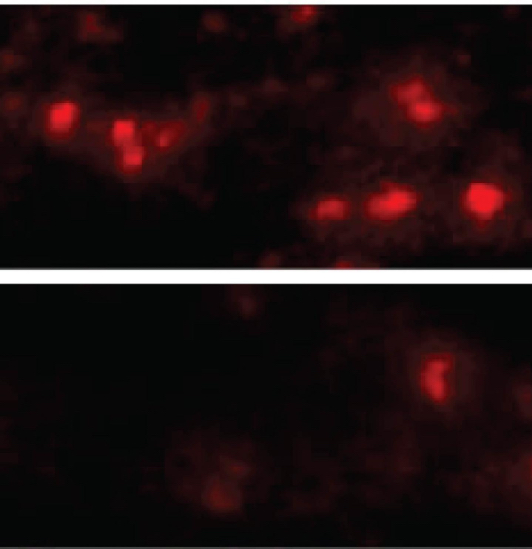
The researchers explain in the release how they knew they were looking for a particular kind of protein called a transmembrane receptor found on the outside of cells. This receptor works sort of like a door lock, admitting only proteins with the right “key.” The investigators initially discovered a type of cell that alpha-synuclein aggregates could not enter — a line of human brain cancer cells grown in a lab. The next step was to add genes one-by-one for transmembrane receptors to the cells and observe whether any would allow the aggregates in, which three of the proteins did, and one of which, LAG3, exhibited a strong preference for latching onto alpha-synuclein aggregates over non-clumped alpha-synuclein.
The next phase of their research was to breed mice lacking the LAG3 gene and to inject them with alpha-synuclein aggregates. Normal mice will develop Parkinson’s-like symptoms soon after being injected, and half of their dopamine-making neurons will die within six months of the injection. However, they found that the mice without the LAG3 protein were almost completely protected from these effects, with antibodies that blocked LAG3 having similar protective effects in cultured neurons.
“We were excited to find not only how alpha-synuclein aggregates spread through the brain, but also that their progress could be blocked by existing antibodies,” says Xiaobo Mao, PhD, a research associate in Dawson’s laboratory and first author of the study.
Dawson said that if LAG3 targeting antibodies currently in clinical trials to test whether they can beef up the immune system during chemotherapy can also be shown to demonstrate the drug’s safety, the process of testing them as Parkinson’s disease therapeutics might be accelerated.
In the meantime, the research team plans to continue testing LAG3 antibodies in mice and further explore LAG3’s functions.